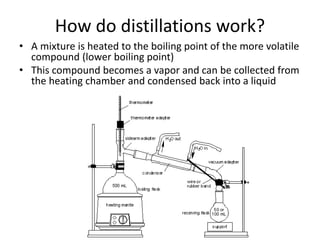
1. The document describes a lab experiment to isolate limonene from orange peels through steam distillation. Peels are blended with water and distilled to obtain an "essential oil" containing limonene, which is then extracted and characterized using gas chromatography.
2. Key steps include grinding orange peels, distilling the peels to obtain limonene, extracting limonene using liquid-liquid extraction, and analyzing the isolated limonene using gas chromatography to determine its boiling point.
3. Steam distillation is used because it allows isolation of limonene at a lower temperature than normal distillation, preventing decomposition of the thermally sensitive terpene compounds like limonene.