1. UV-Vis spectroscopy detects electronic transitions in molecules when photons are absorbed, promoting electrons to higher energy states. Transitions involve π or n electrons and occur in the 200-700nm region.
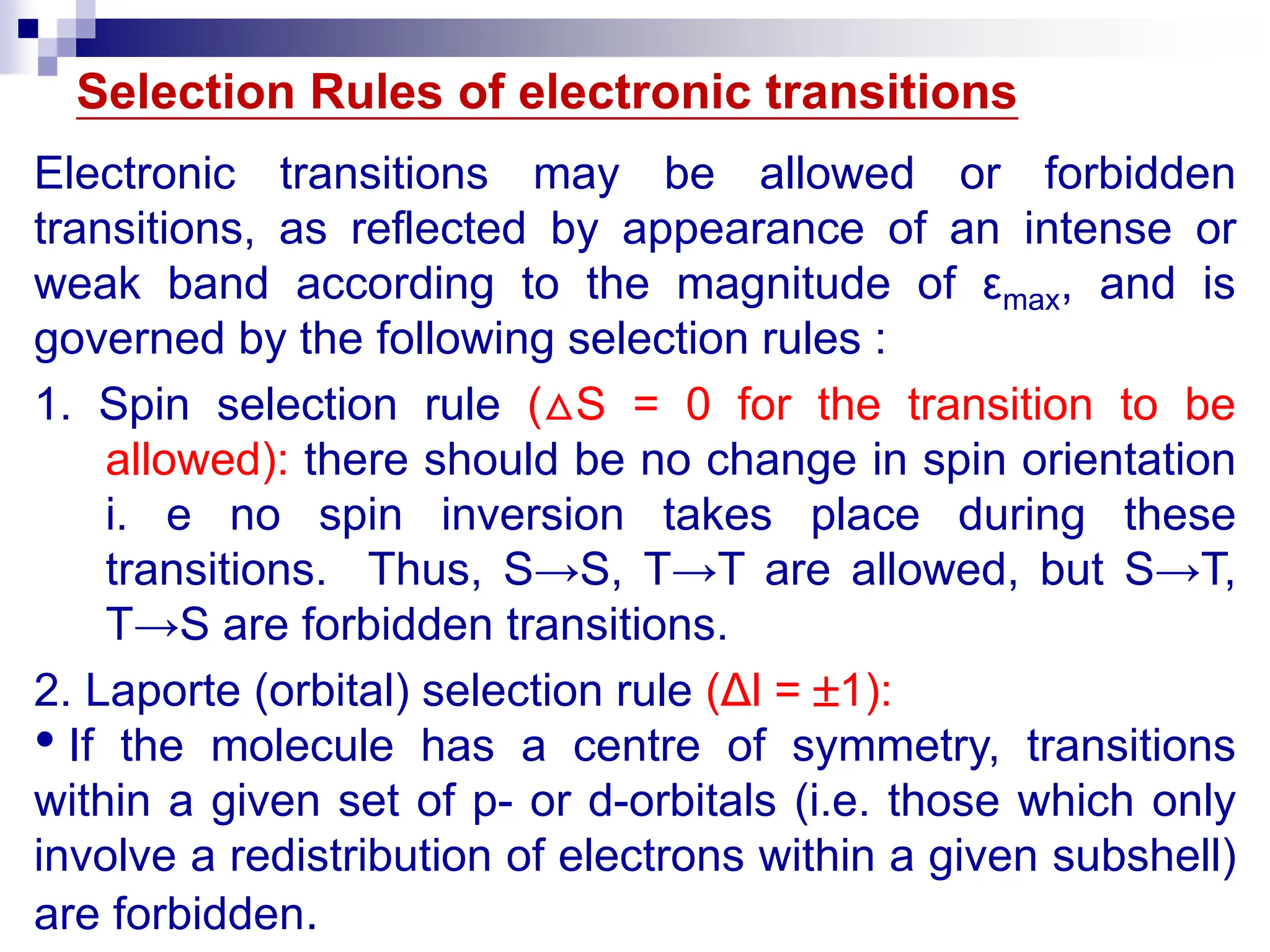
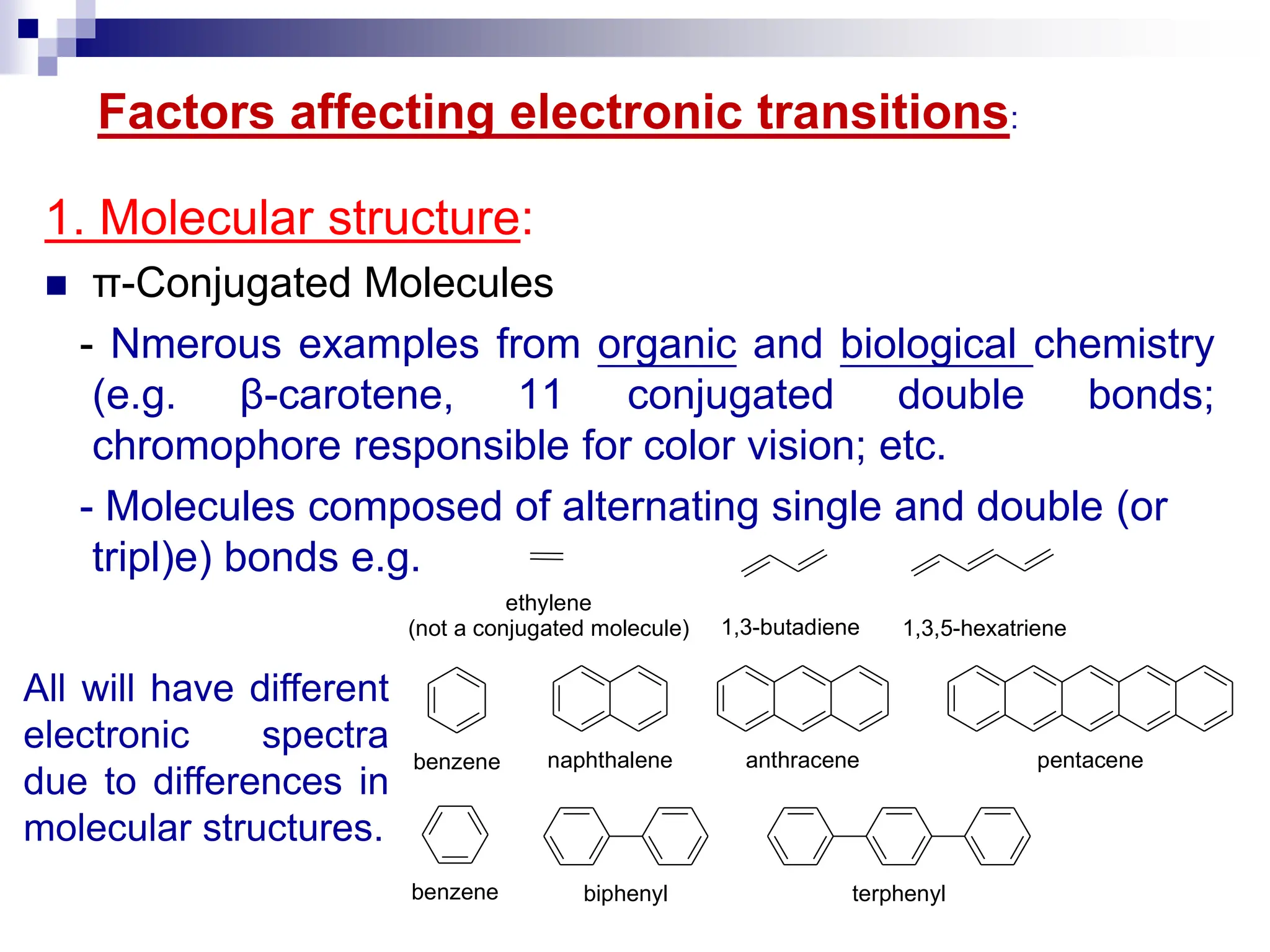
2. Absorption depends on functional groups called chromophores as well as conjugation, solvent effects, and molecular structure. Selection rules govern allowed transitions.
3. Spectra appear as bands representing many overlapping transitions between vibrational/rotational energy levels of ground and excited electronic states. Band features provide structural information.