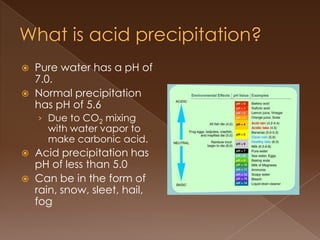
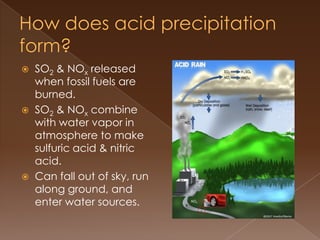
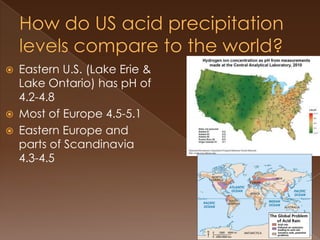
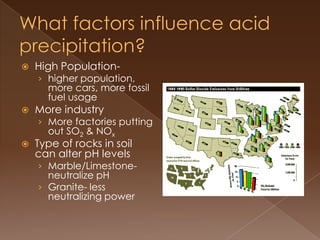
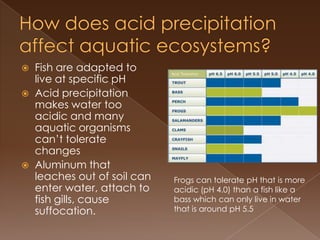
Acid precipitation forms when sulfur dioxide and nitrogen oxides from fossil fuel combustion react with water vapor to form acids. This acidic precipitation damages plants by leaching nutrients from soil and releasing aluminum, harms aquatic life by making waters too acidic for fish and frogs, and affects humans through respiratory irritation, loss of commercial fishing, and building corrosion. Countries have signed agreements to reduce emissions that cross borders and cause acid precipitation in downwind nations.