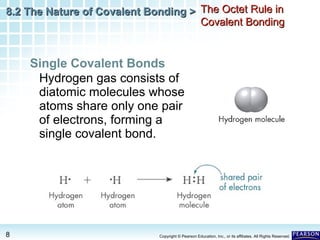
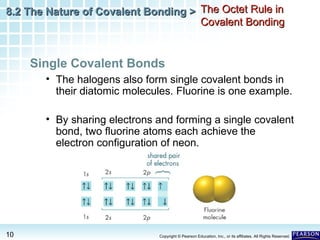
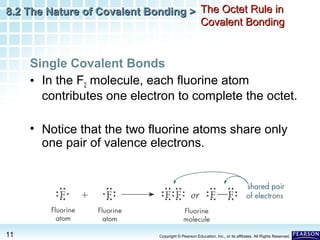
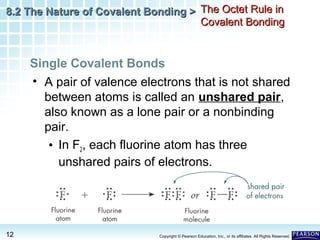
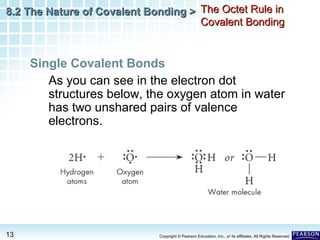
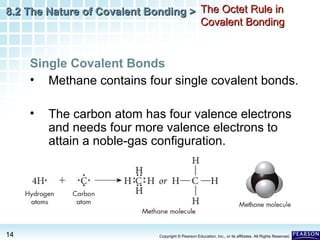
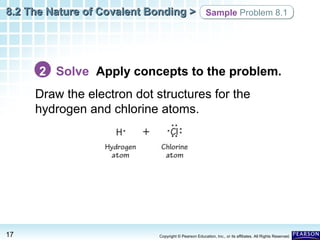
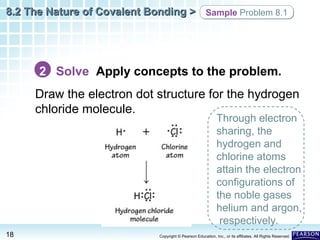
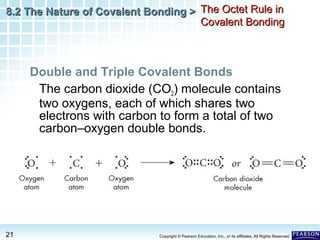
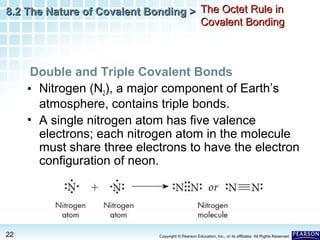
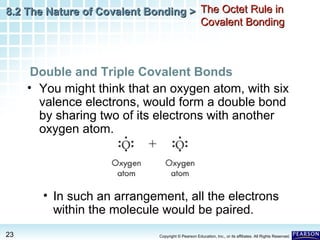
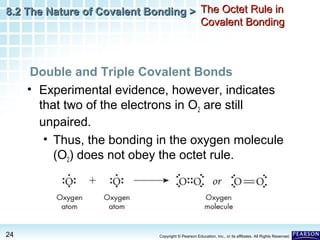
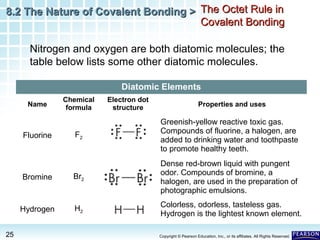
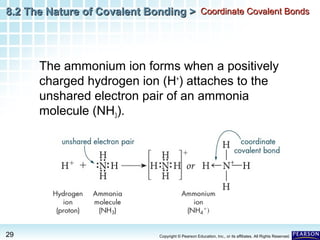
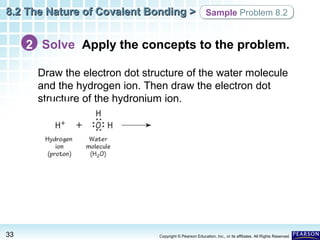
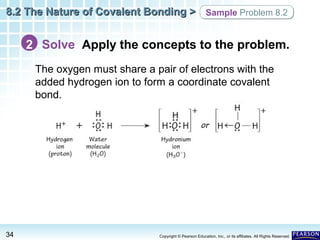
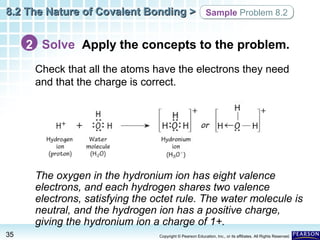
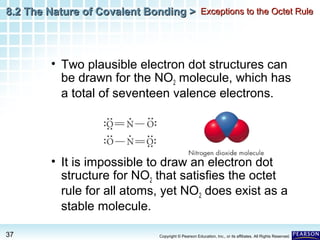
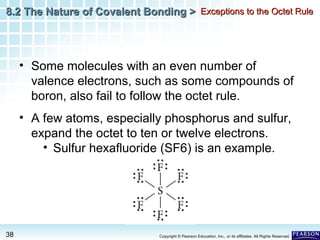
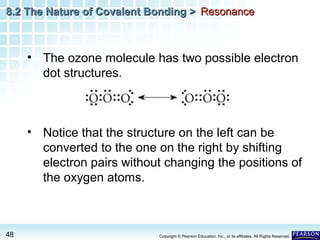
The document discusses the octet rule in covalent bonding. It states that in covalent bonds, electron sharing usually occurs so that atoms attain the electron configuration of noble gases, with eight electrons in their outer shell. Atoms can form single, double or triple bonds to reach an octet. Single bonds involve one shared pair of electrons, double bonds two shared pairs, and triple bonds three shared pairs. Examples like hydrogen gas, oxygen gas, carbon dioxide and nitrogen gas are provided to illustrate the different bond types. The octet rule explains the bonding in many covalent compounds composed of nonmetals.