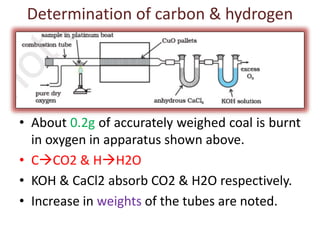
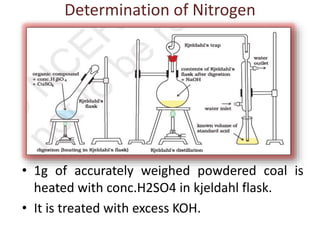
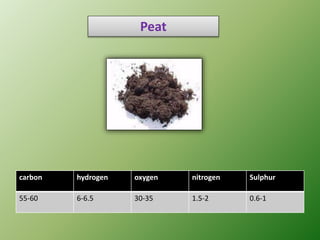
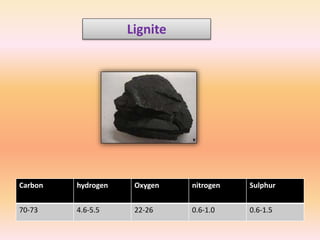
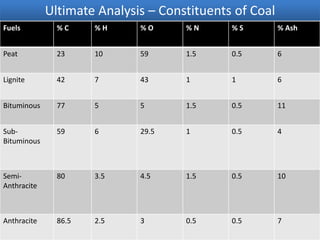
The document discusses the ultimate analysis of coal, detailing its composition and the methods for determining the content of carbon, hydrogen, nitrogen, sulfur, and oxygen. It highlights the importance of understanding these constituents for evaluating the utility of coal as a fossil fuel and its applications in energy production. The analysis distinguishes between proximate and ultimate methods and provides specific procedures for obtaining the necessary measurements.