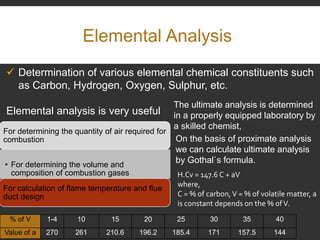
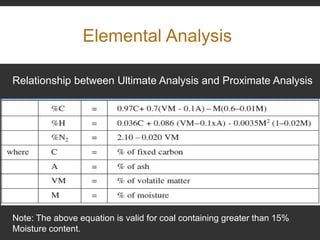
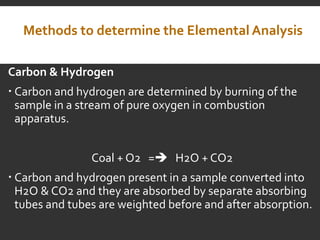
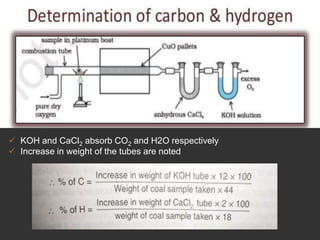
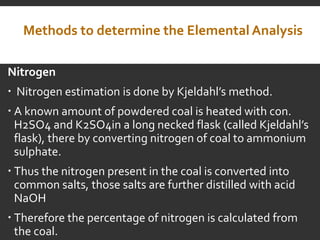
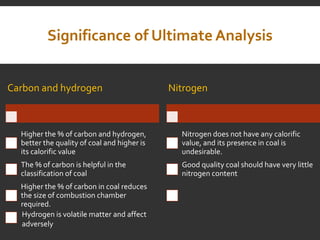
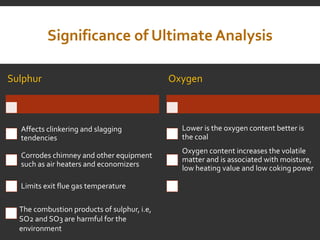
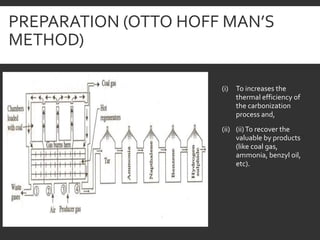
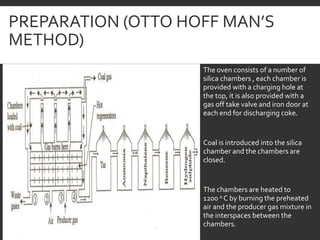
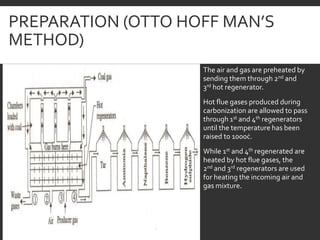
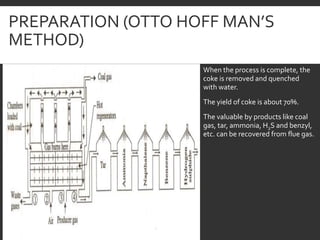
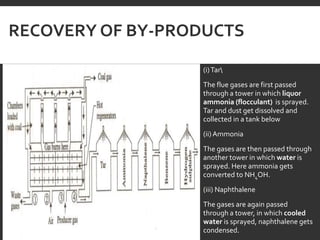
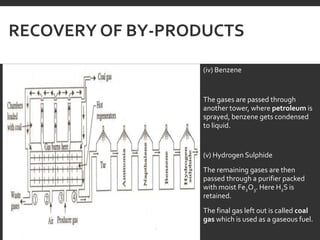
Elemental analysis determines the chemical constituents of materials like carbon, hydrogen, oxygen, and sulfur. It is useful for determining combustion properties and flue gas composition. The analysis involves laboratory techniques like combustion analysis for carbon and hydrogen, Kjeldahl's method for nitrogen, and bomb calorimetry for sulfur. Elemental analysis provides information on coal quality and classification. Carbonization is the heating of coal in the absence of oxygen to produce coke. Otto Hoffman's carbonization method allows for byproduct recovery like coal gas, tar, and ammonia through controlled heating and gas purification. Metallurgical coke must have high purity, porosity, strength, and heating value to serve as a good fuel in blast furn