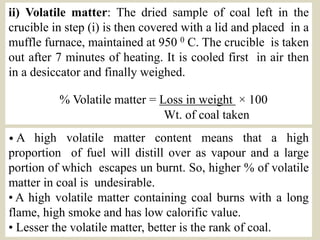
The document discusses the analysis of solid fuels such as coal. It describes the proximate and ultimate analysis of coal. The proximate analysis determines the moisture, volatile matter, ash and fixed carbon content. This gives information on the coal's classification and suitability for industrial use. The ultimate analysis determines the carbon, hydrogen, sulfur, nitrogen, oxygen and ash content. This is needed to calculate the heat output of coal. Several examples are given to show calculations for determining the percentage composition of elements like sulfur and nitrogen in a coal sample.