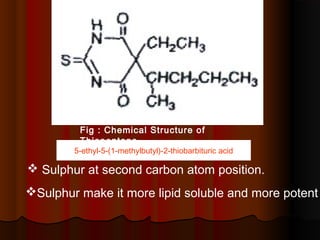
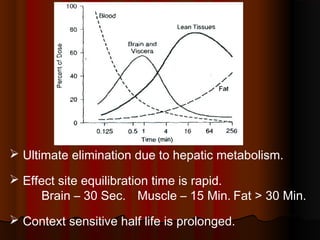
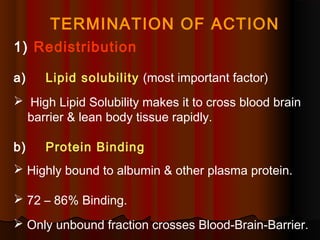
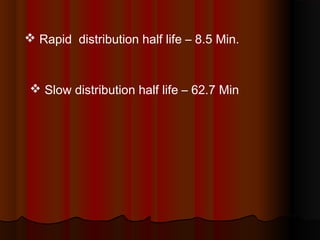
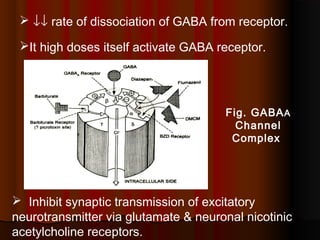
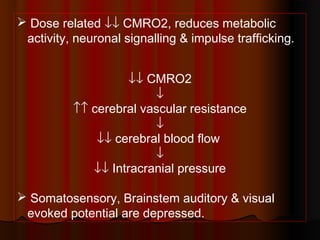
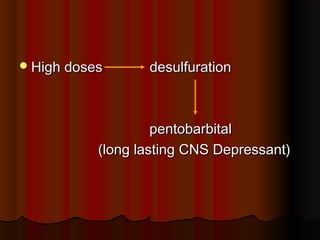
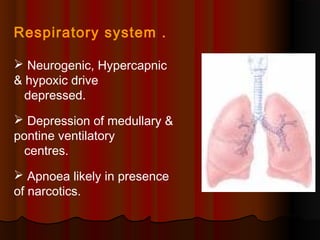
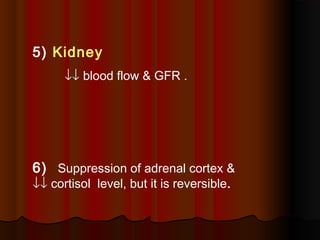
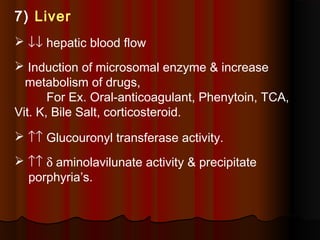
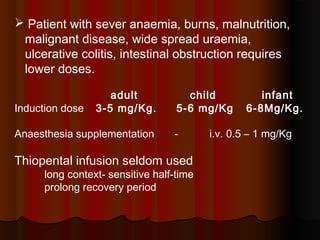
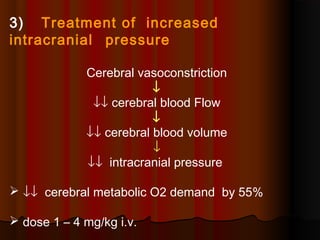
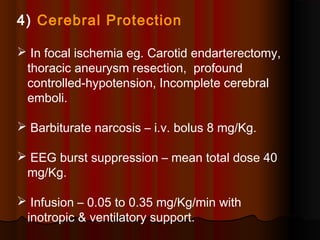
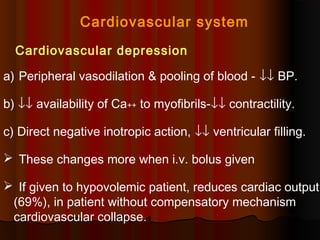
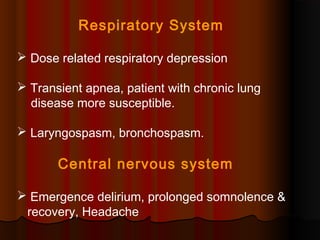
Thiopentone is an ultra short-acting barbiturate used for induction of anesthesia. It works by enhancing the effect of the inhibitory neurotransmitter GABA at GABAA receptors in the brain, causing sedation, hypnosis and general anesthesia. It has a rapid onset of 10-20 seconds when given intravenously and is redistributed and metabolized quickly, typically causing awakening within 5-15 minutes. Common uses include induction of anesthesia and treatment of increased intracranial pressure. Side effects are generally mild and related to its cardiovascular and respiratory depressant effects.