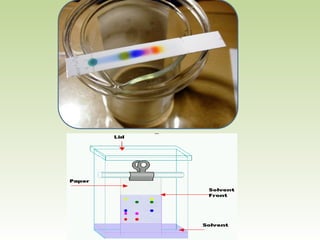
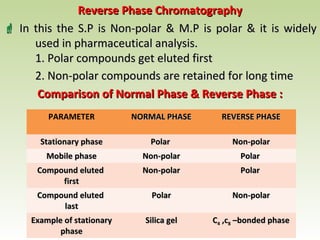
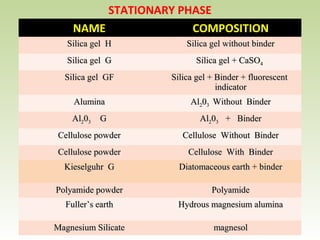
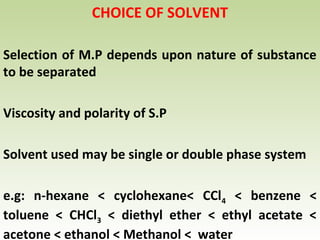
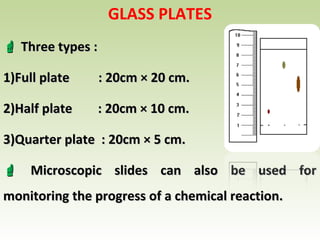
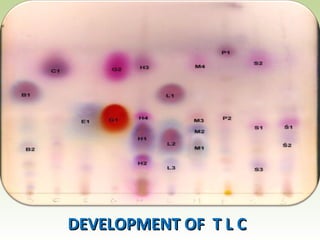
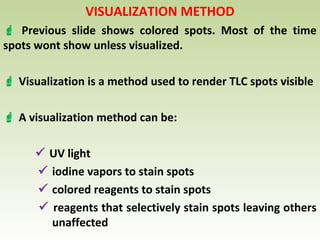
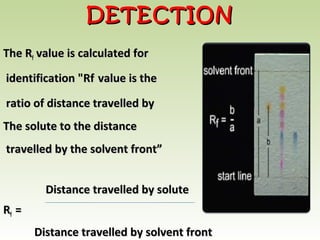
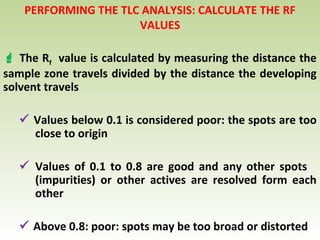
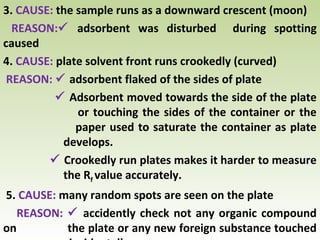
Thin layer chromatography (TLC) is a technique used to separate chemical compounds. It involves a stationary phase, consisting of a thin layer of adsorbent material like silica gel coated on a flat surface, and a mobile phase of solvent that travels up the stationary phase via capillary action. TLC is useful for determining and detecting pigments, pesticides, insecticides, and for identifying compounds in forensic analysis and chemical reactions. It requires small amounts of sample and solvent and allows for fast separation of compounds.