Embed presentation
Download to read offline


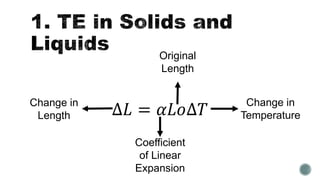




Most matter expands when heated and contracts when cooled. The volume of a material generally increases as its temperature increases, with the fractional change in length or volume defined as the coefficient of linear expansion. Charles' Law states that for a given temperature change, all gases expand by the same amount of volume, while Boyle's Law says the volume of a gas varies inversely with pressure if temperature is held constant. The General Gas Law combines these, relating the pressure, volume, and temperature of a gas.






