This document provides an overview of concepts related to temperature, expansion, and the ideal gas law. It discusses:
- Brownian motion and how it helped establish that everything is made of atoms.
- Measurements of atomic size and mass.
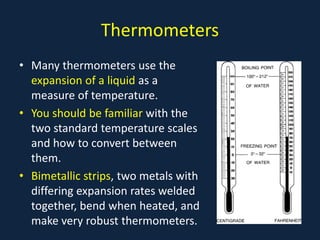
- Early thermometers developed by Galileo using liquid expansion.
- Thermal expansion of solids and liquids, defined by coefficients of linear and volume expansion.
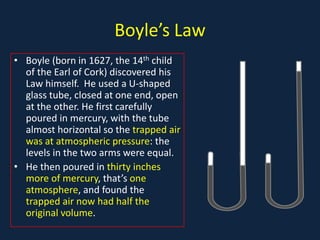
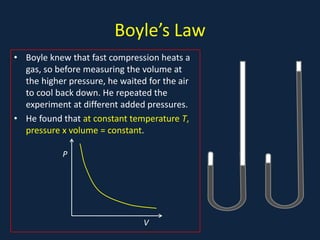
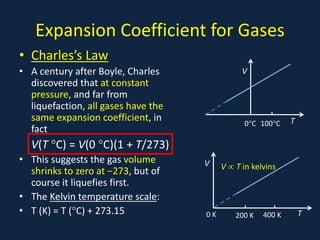
- Gas laws including Boyle's law relating pressure and volume at constant temperature, Charles's law relating volume and temperature at constant pressure, and the combined ideal gas law.