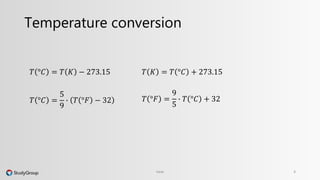
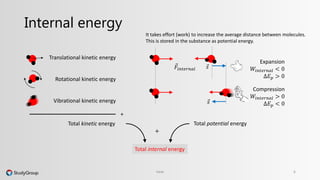
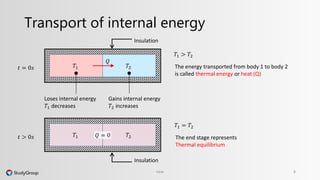
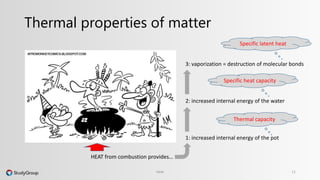
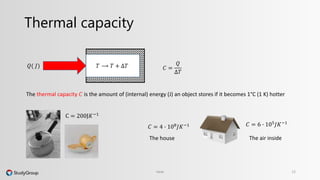
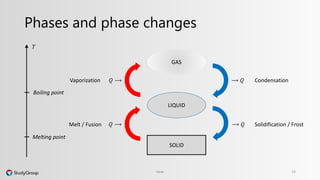
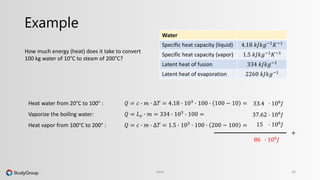
The document discusses various topics related to heat and temperature. It defines different temperature scales including Fahrenheit, Celsius, and Kelvin. It explains how to convert between temperature scales using formulas. It describes concepts such as thermal capacity, specific heat capacity, phases of matter, phase changes, and latent heats. An example problem demonstrates how to calculate the heat required to change the phase of water from liquid to vapor.