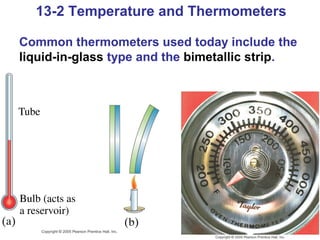
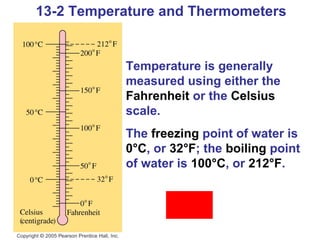
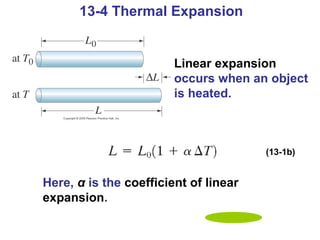
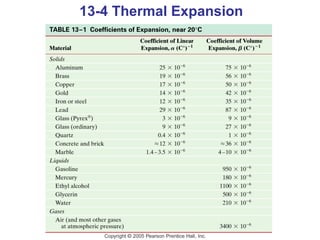
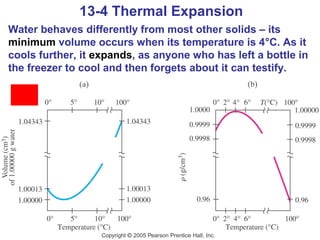
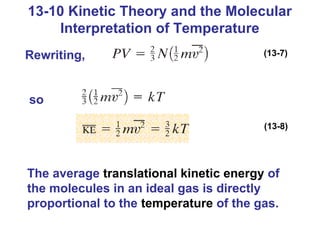
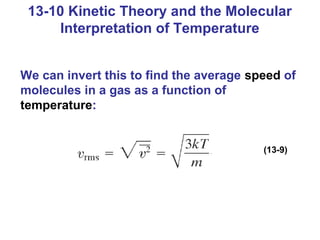
This document summarizes key concepts about temperature, thermometers, thermal expansion, the ideal gas law, and kinetic theory. It explains that temperature is a measure of how hot or cold something is, and most materials expand when heated. Thermometers measure temperature by taking advantage of a property of matter that changes with temperature, such as the expansion of liquid in a glass tube. The ideal gas law relates pressure, volume, temperature, and amount of gas. Kinetic theory interprets temperature at the molecular level as a measure of the average kinetic energy of gas particles, which increases with temperature.