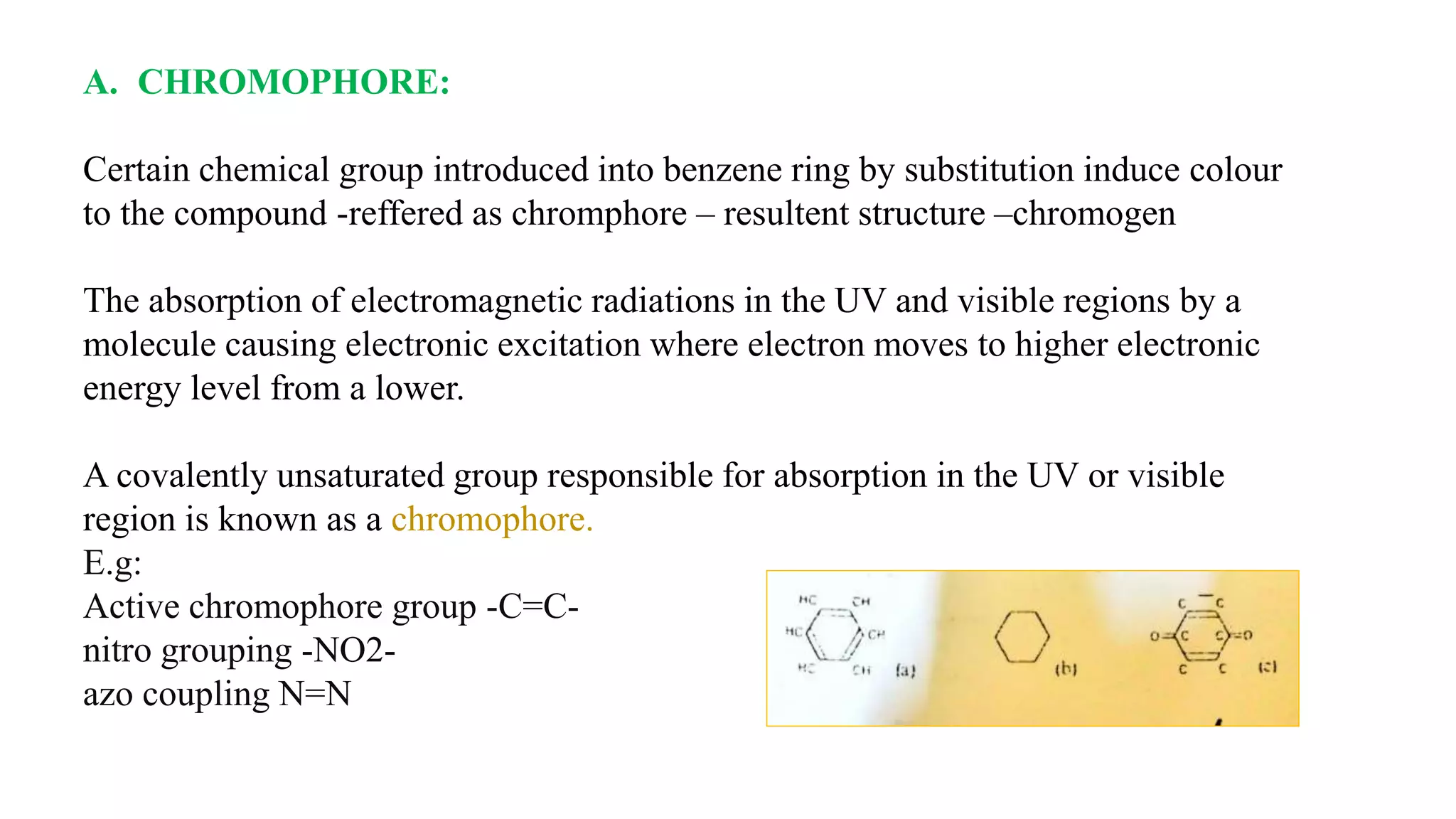
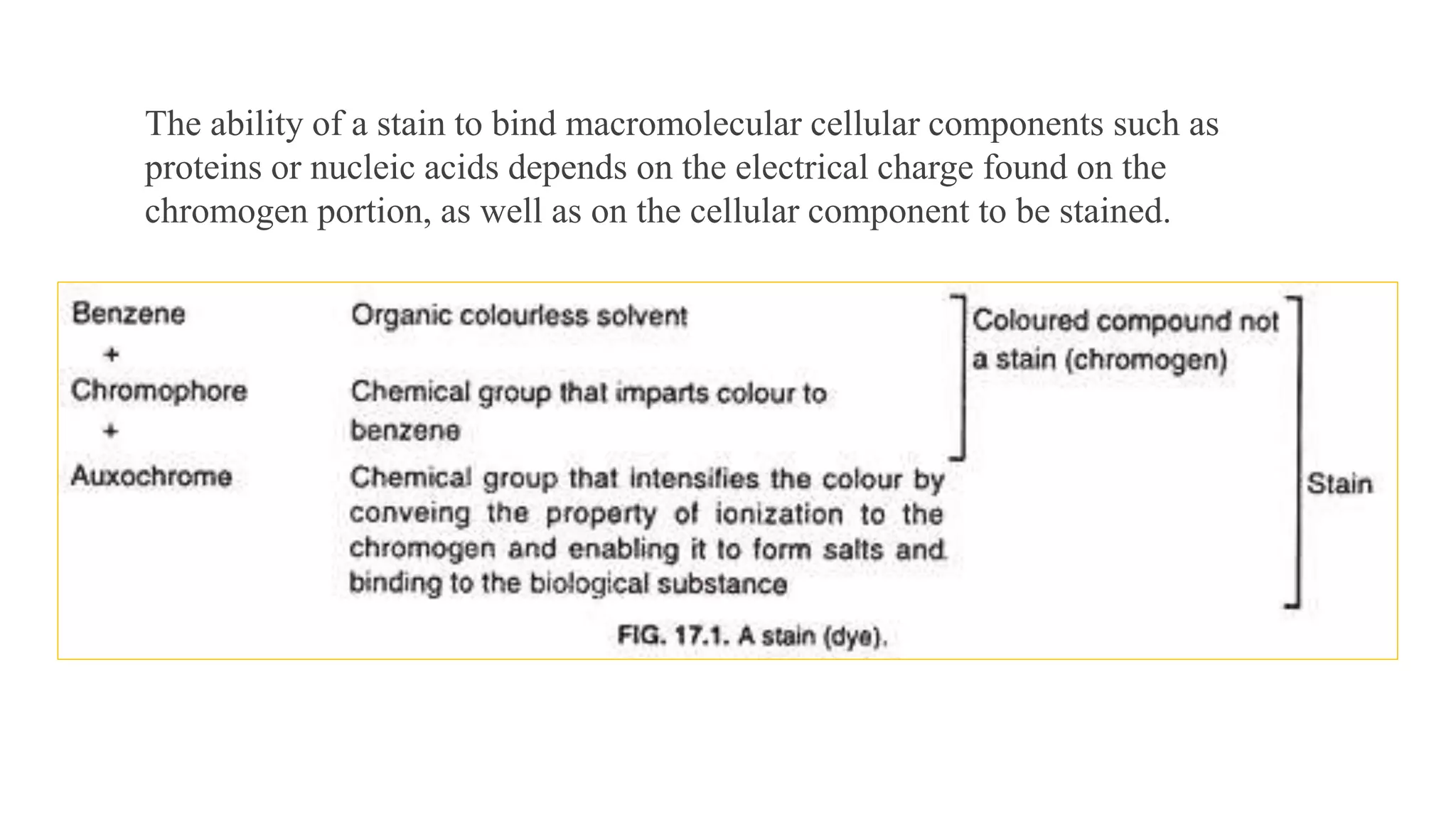
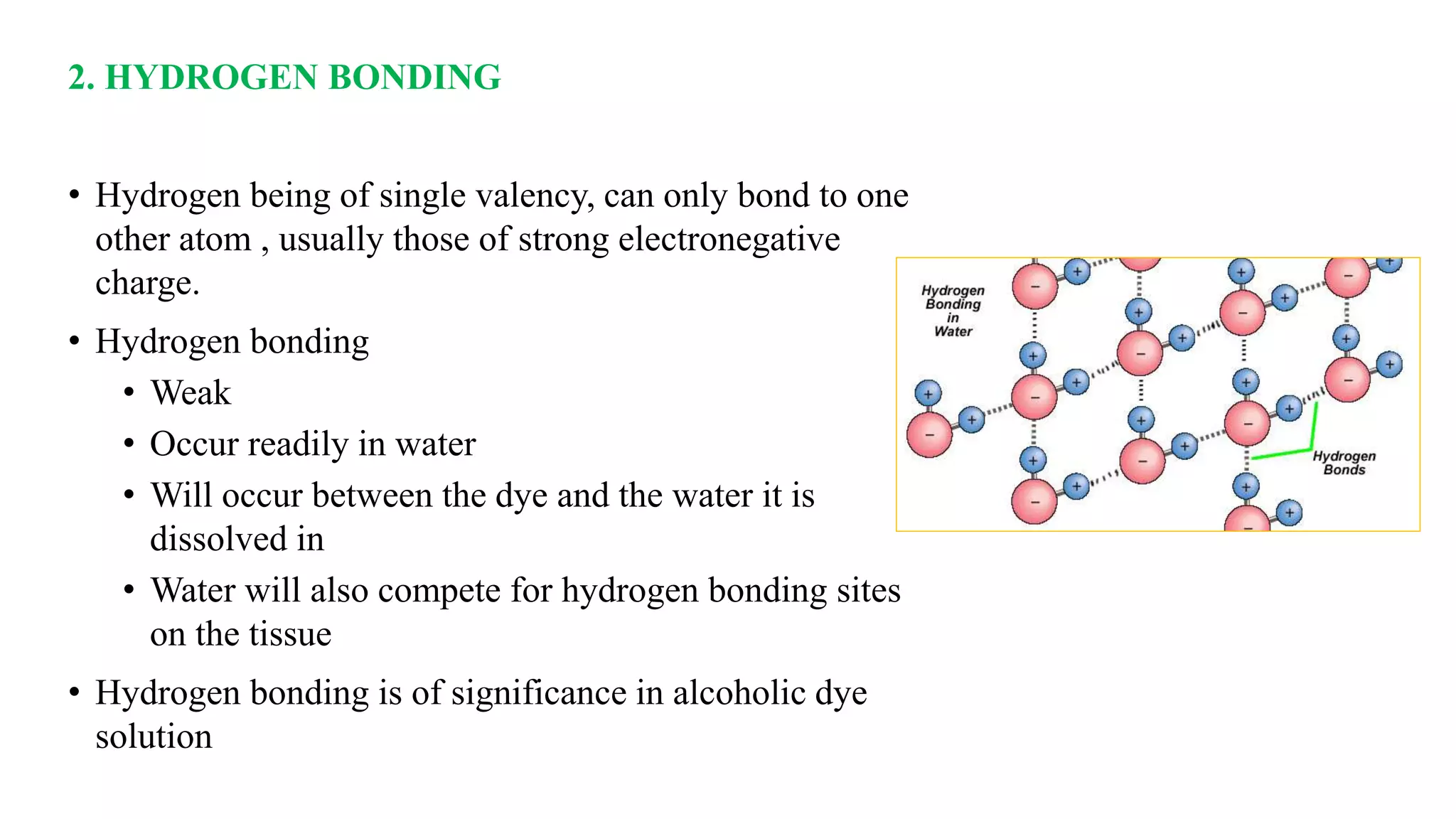
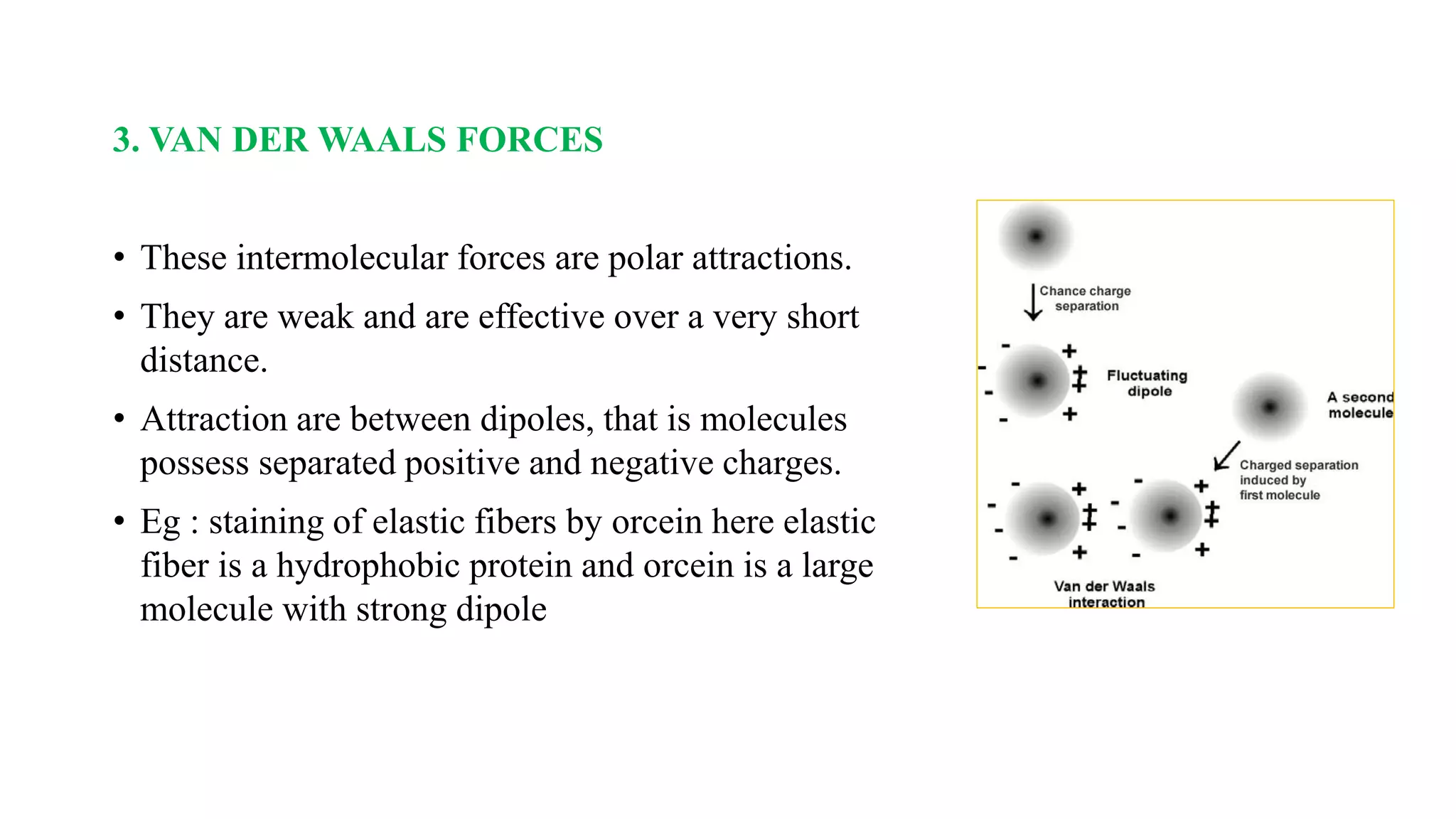
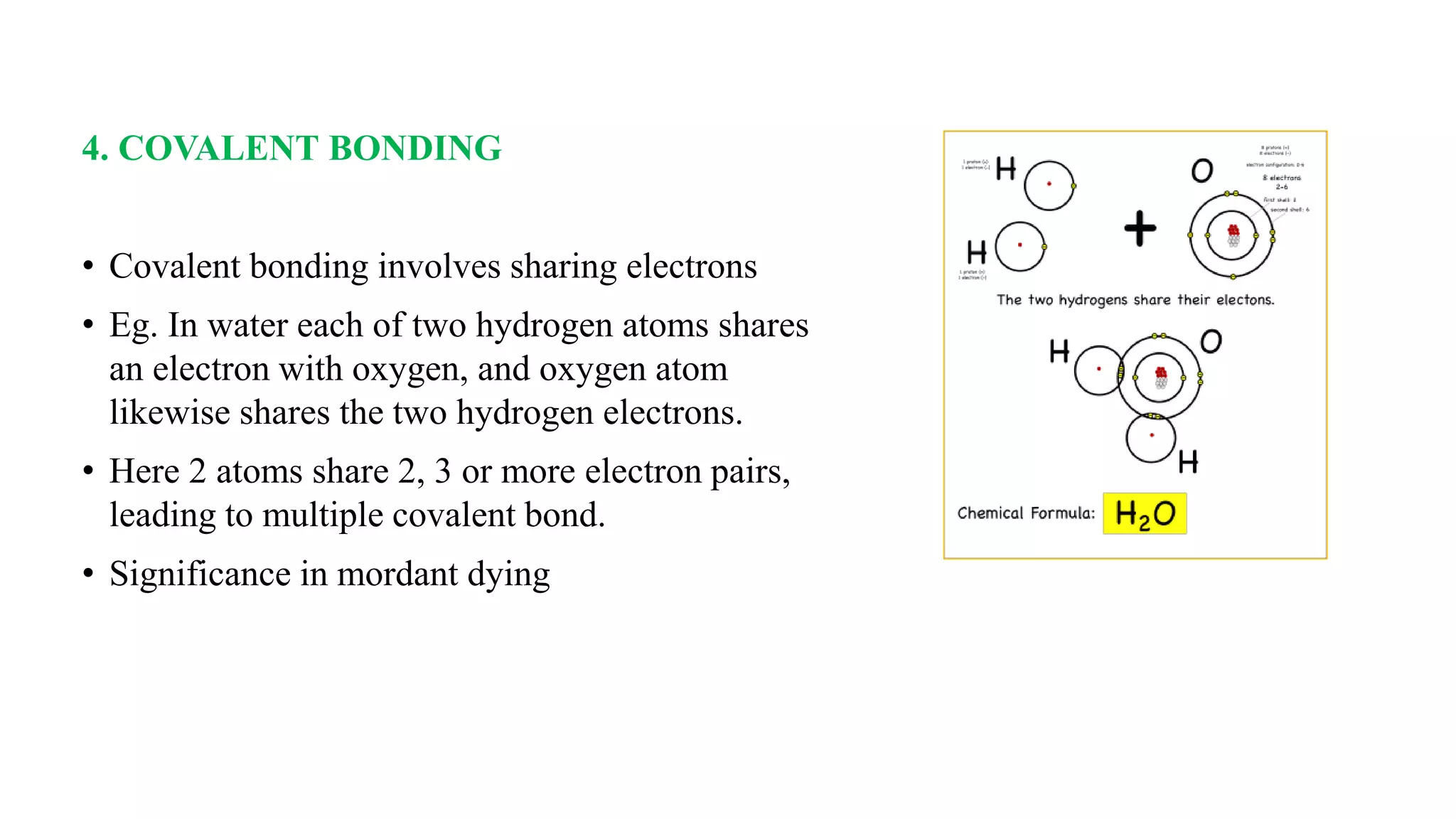
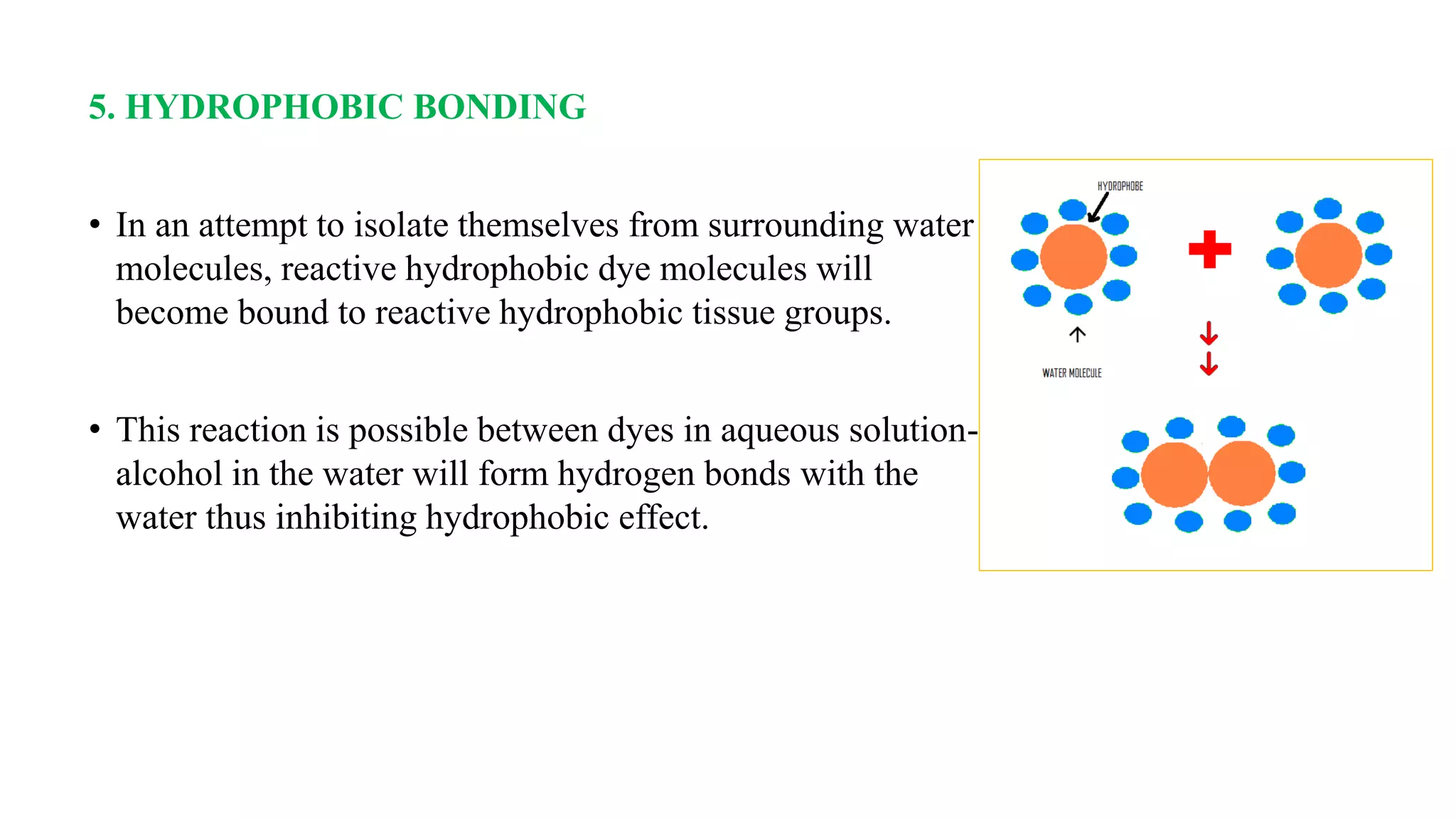
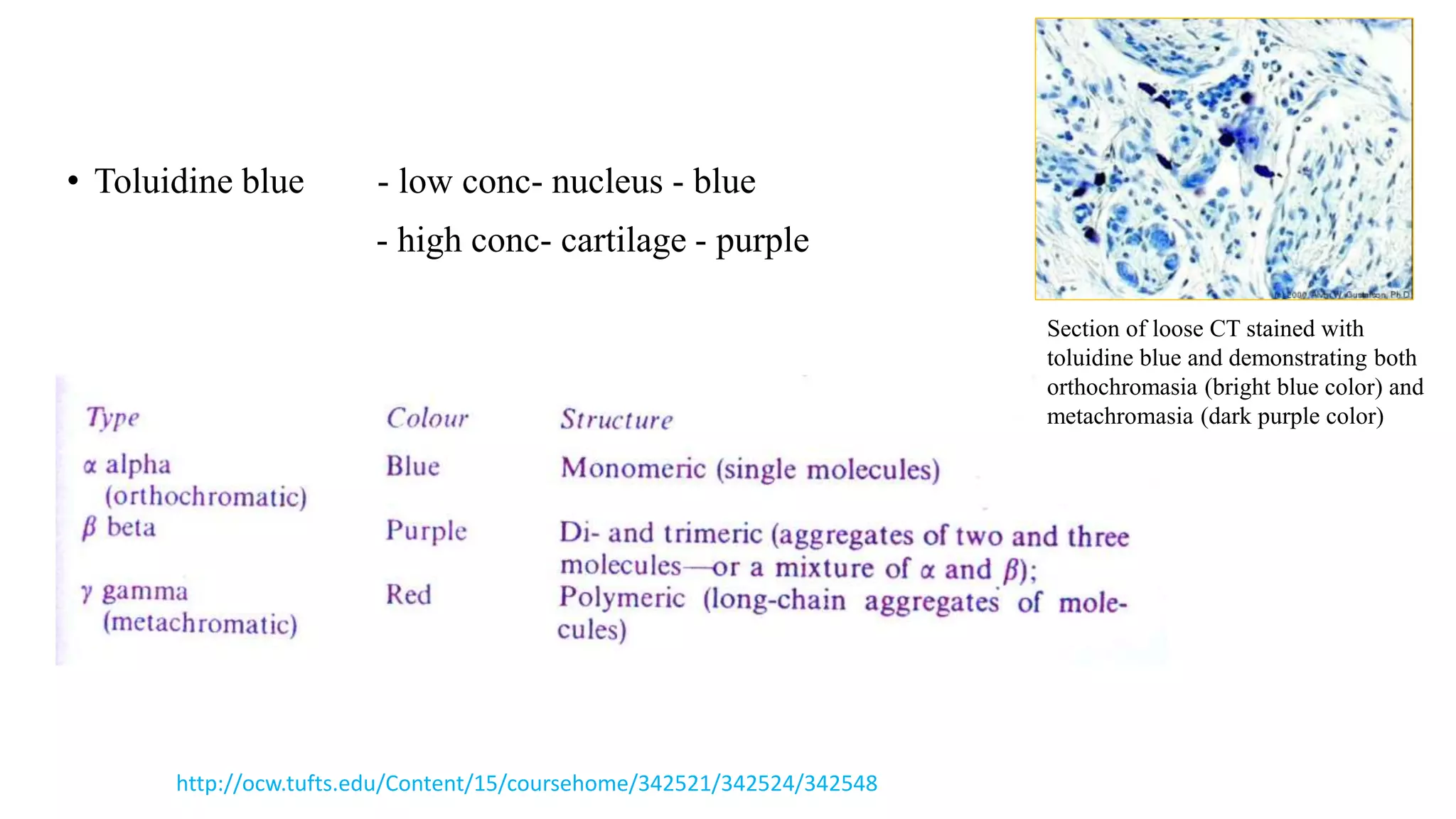
The document discusses the theories and mechanisms of biological staining, including the nature of stains, types, and classification of dyes. It details the chemical structure of stains, including chromophore and auxochrome groups, and explores how dyes interact with tissue through various bonding types. Additionally, it covers the staining process used in histology, specifically for paraffin sections, and the role of mordants and mounting media in tissue preparation.