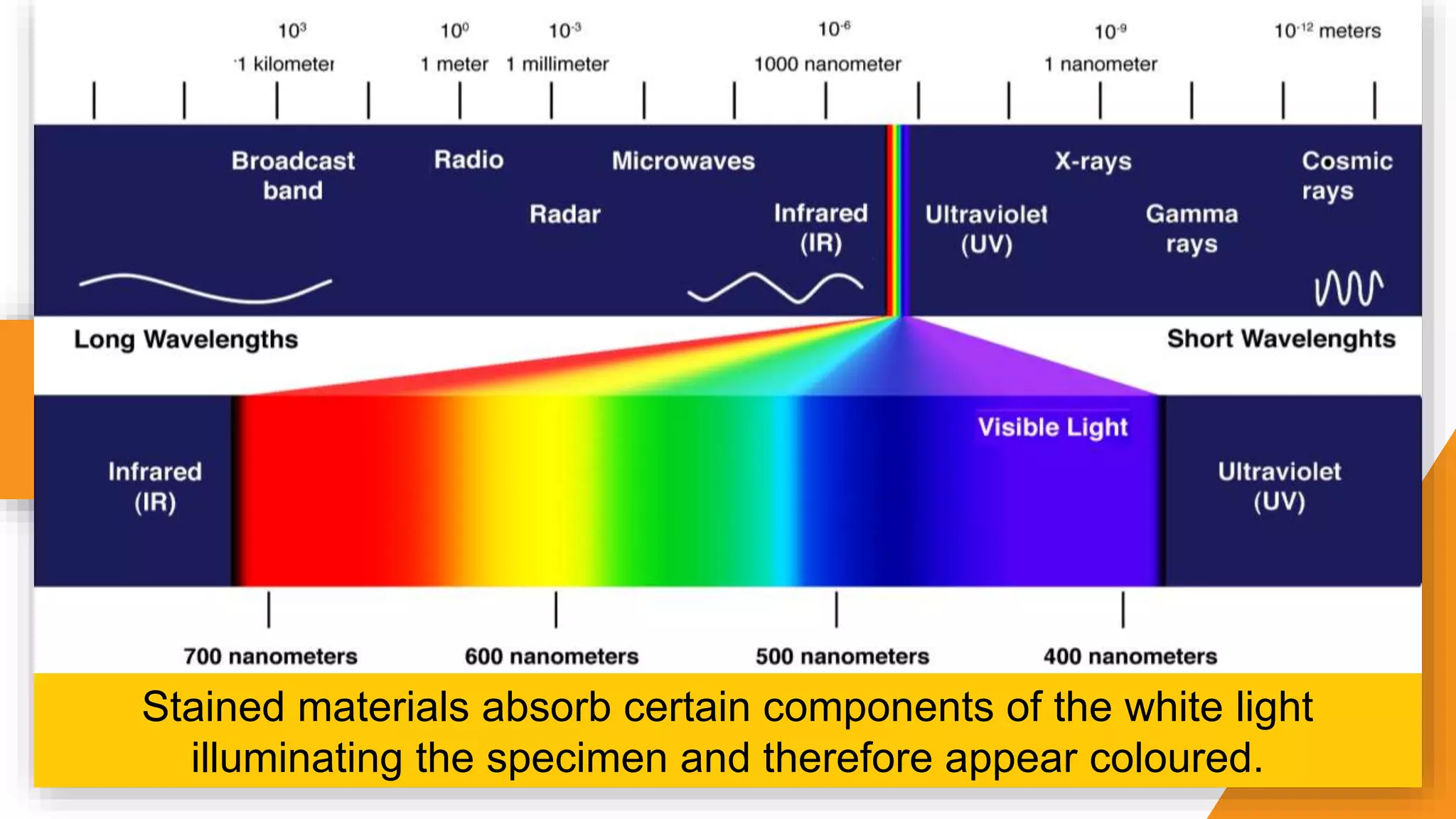
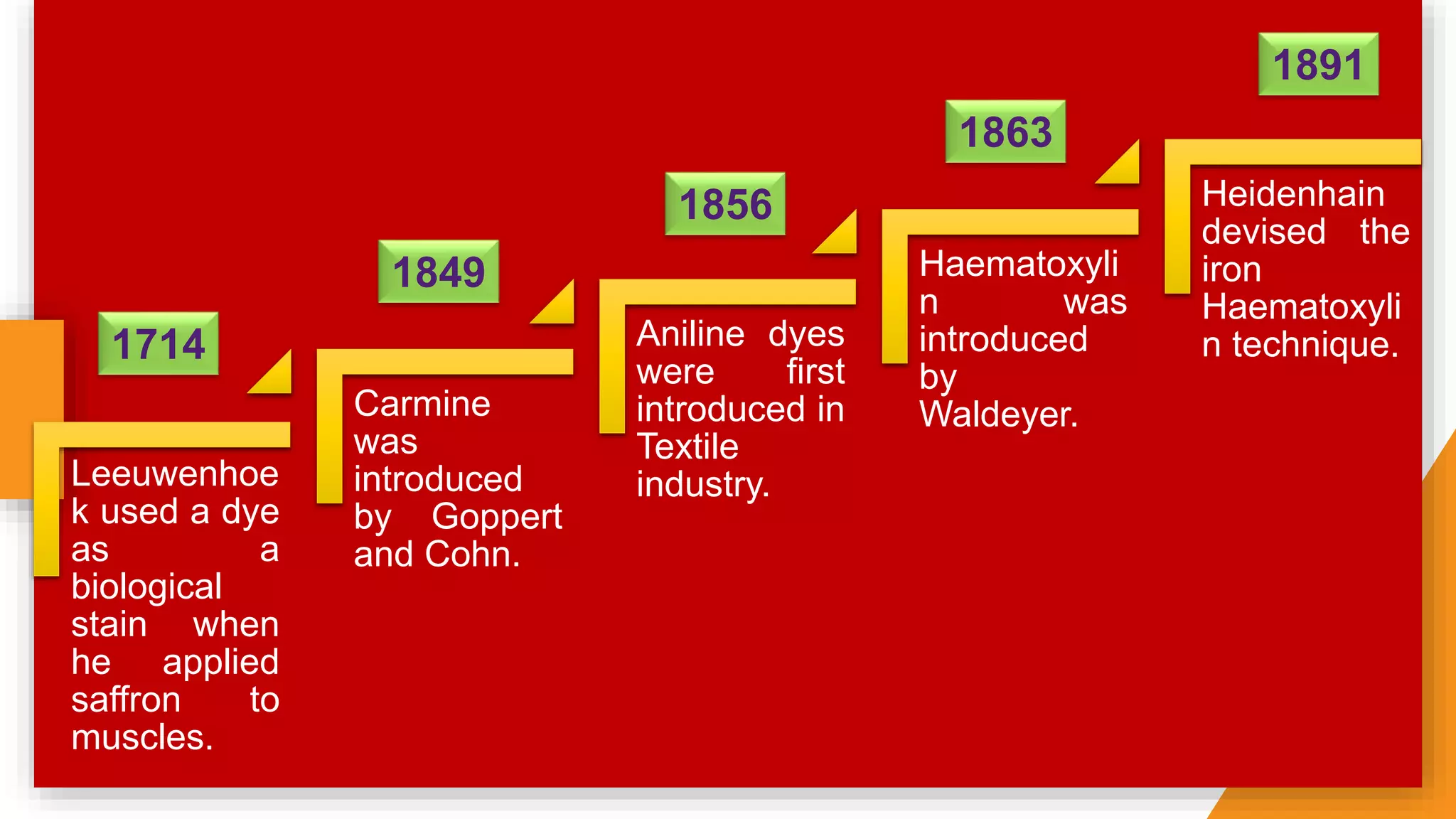
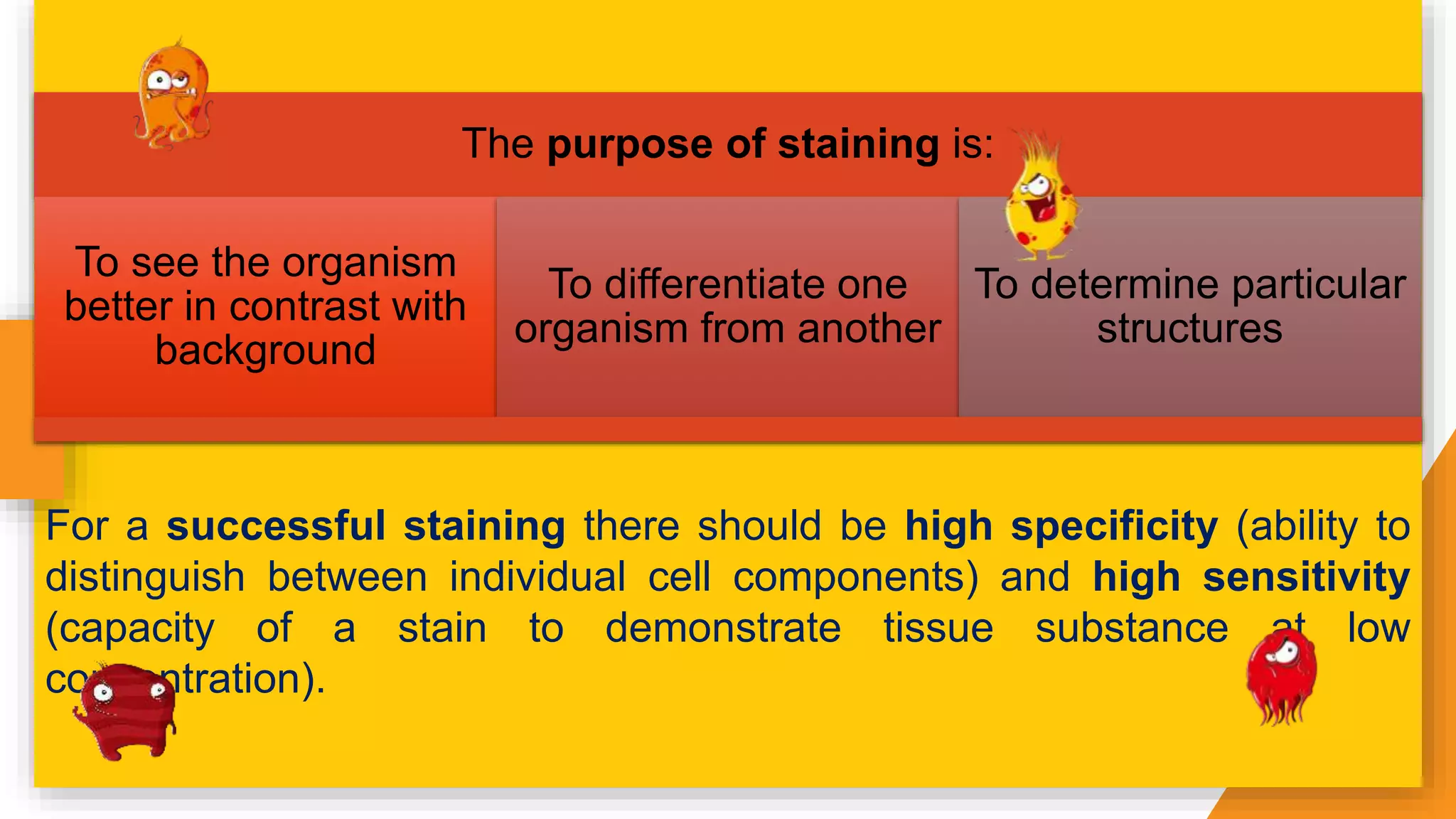
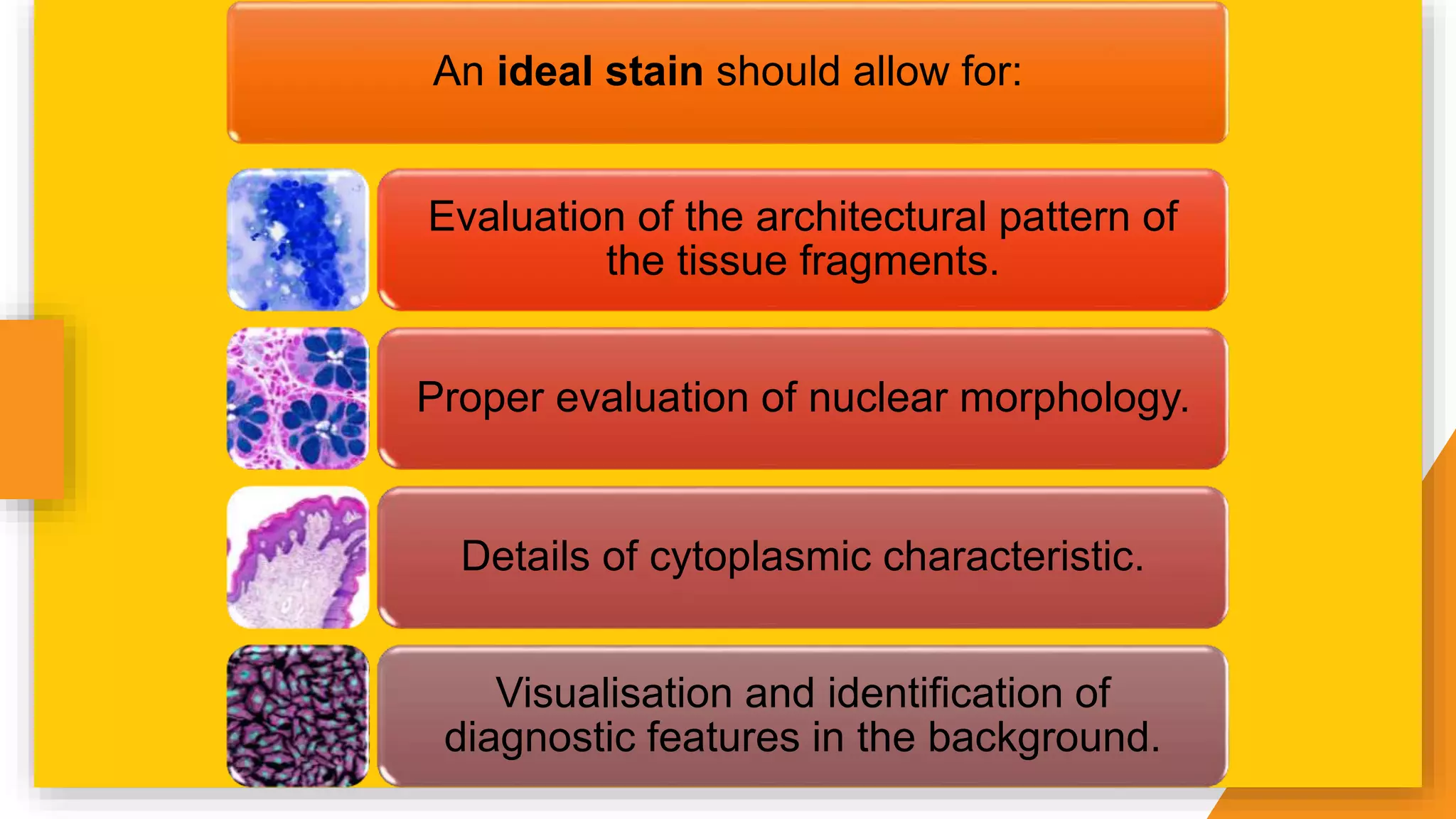
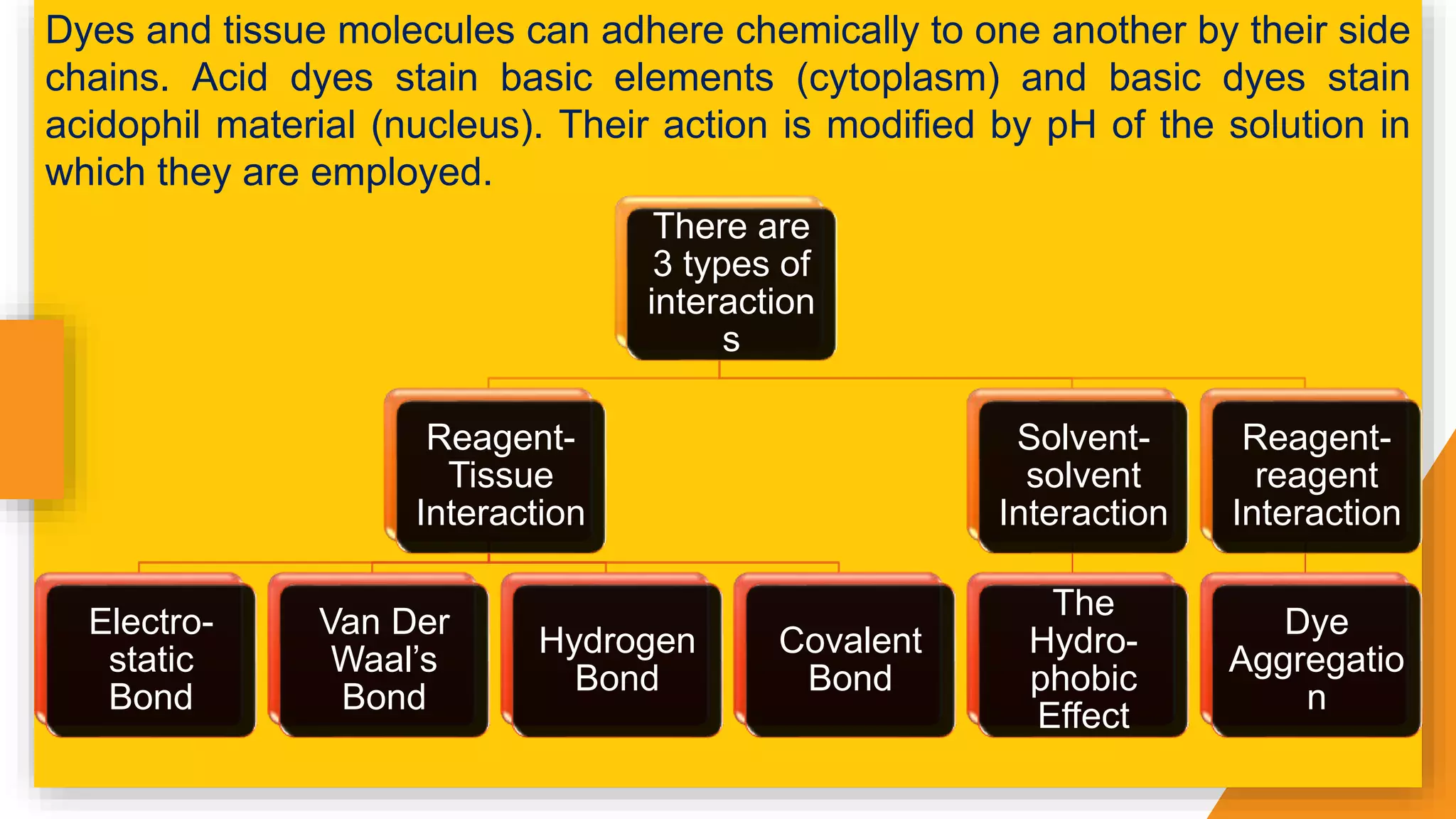
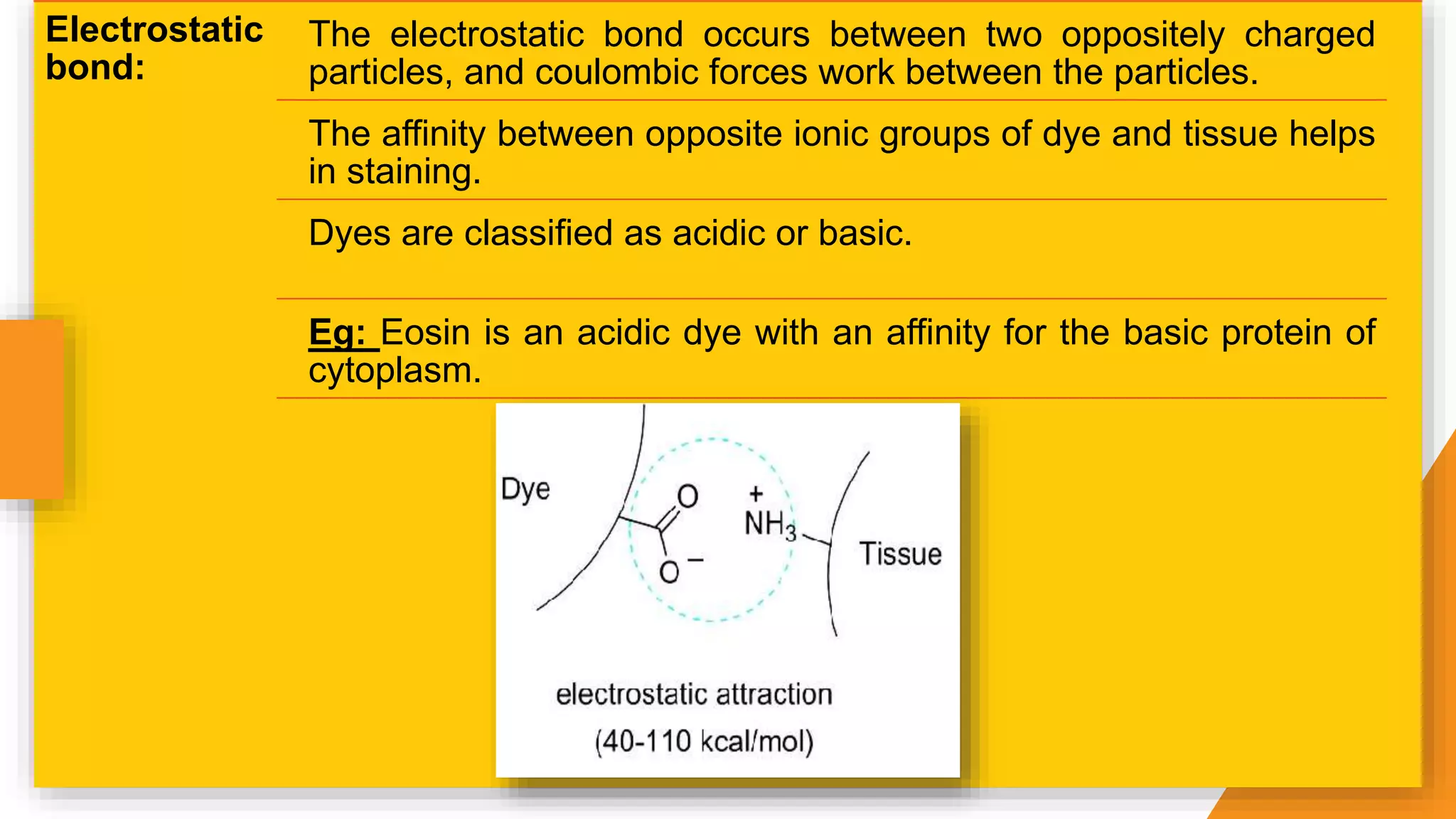
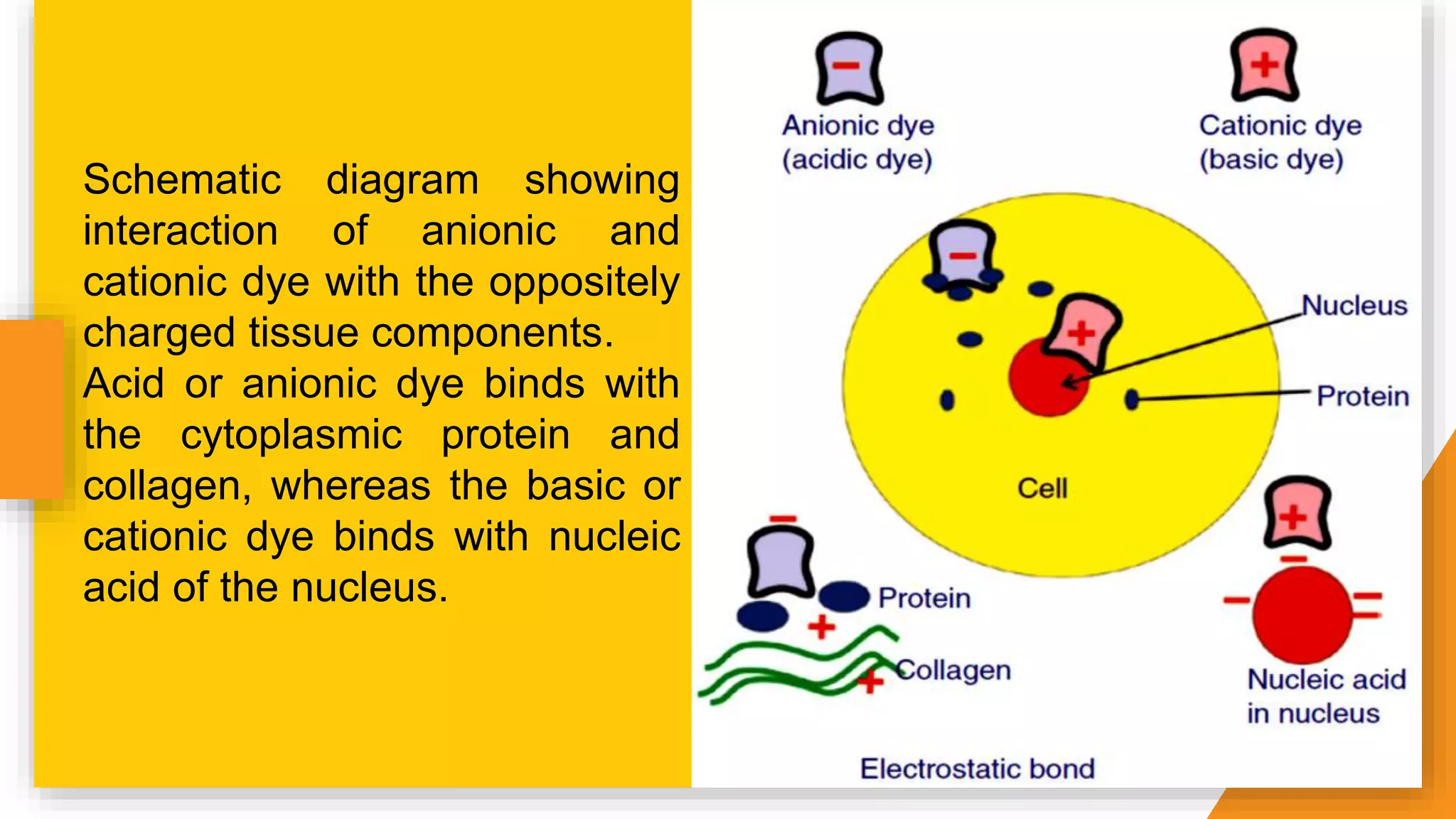
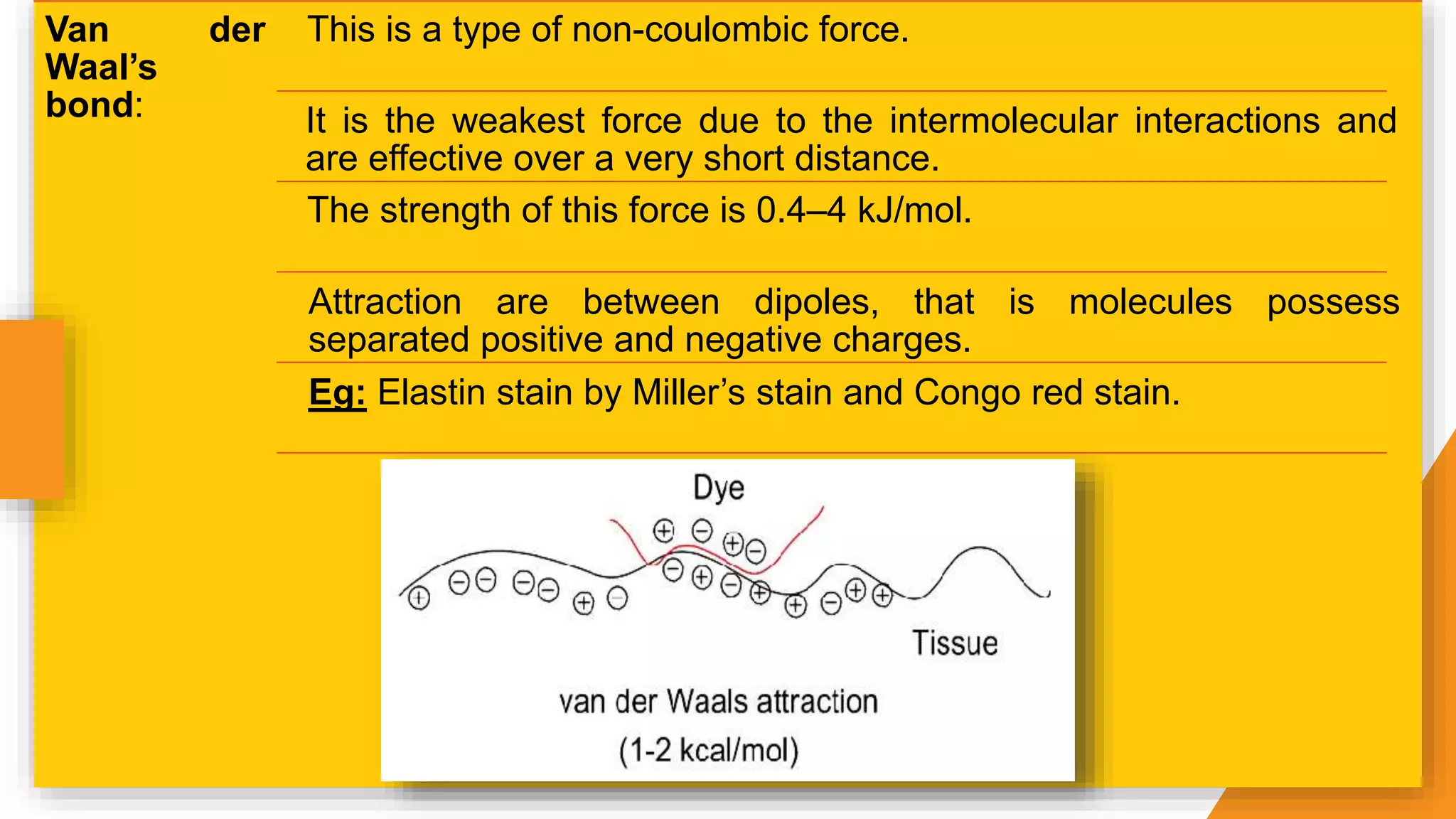
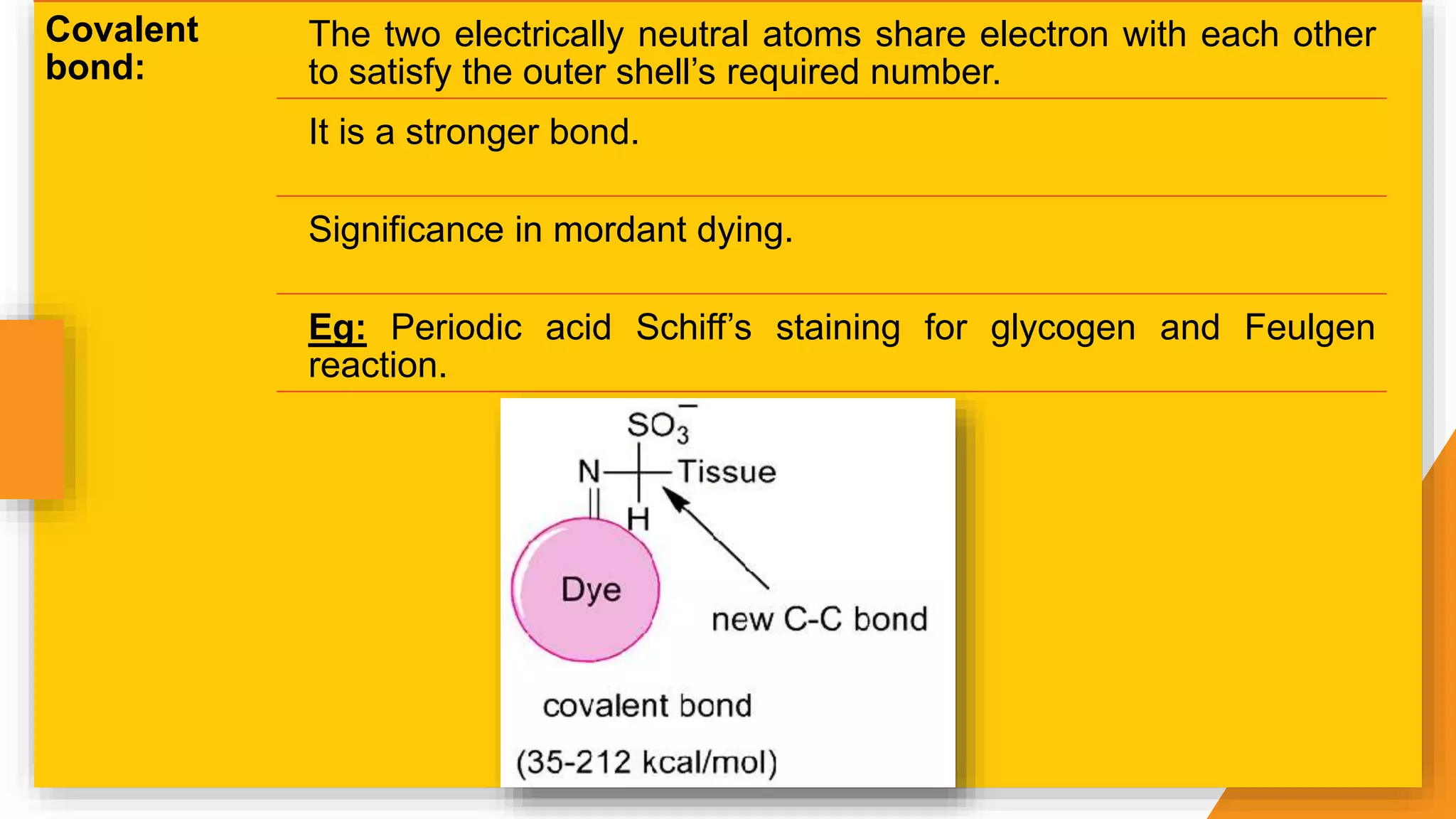
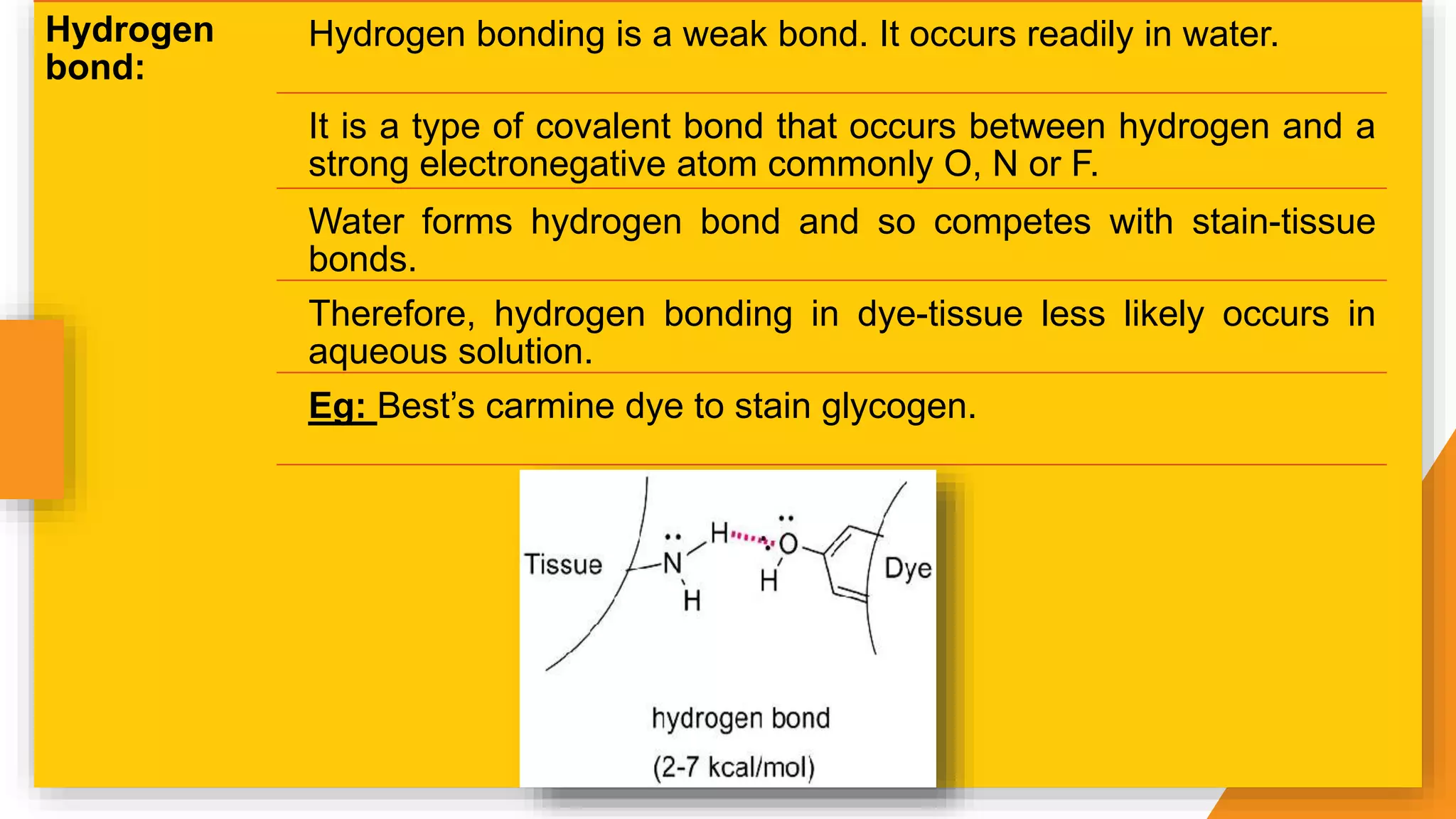
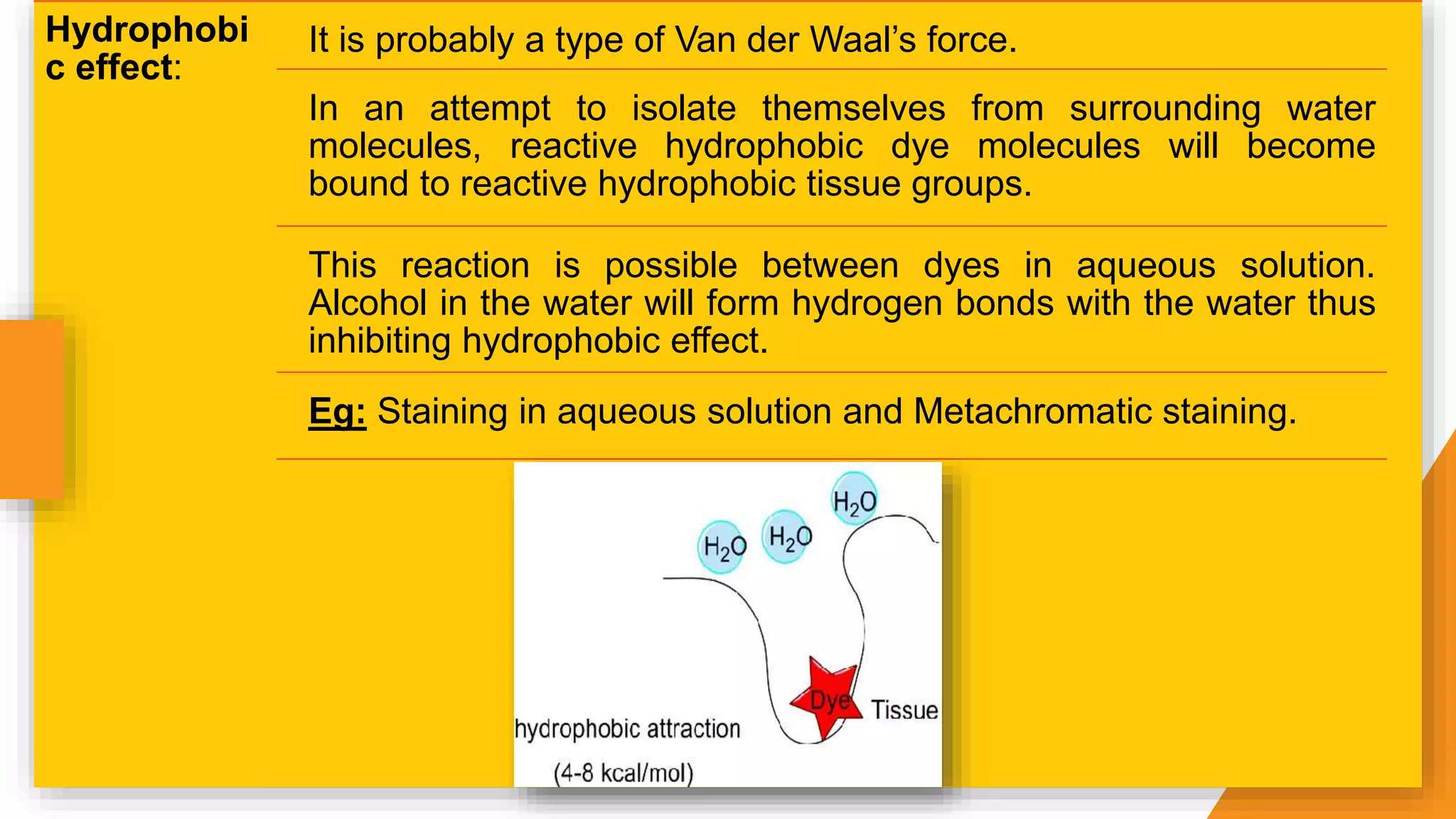
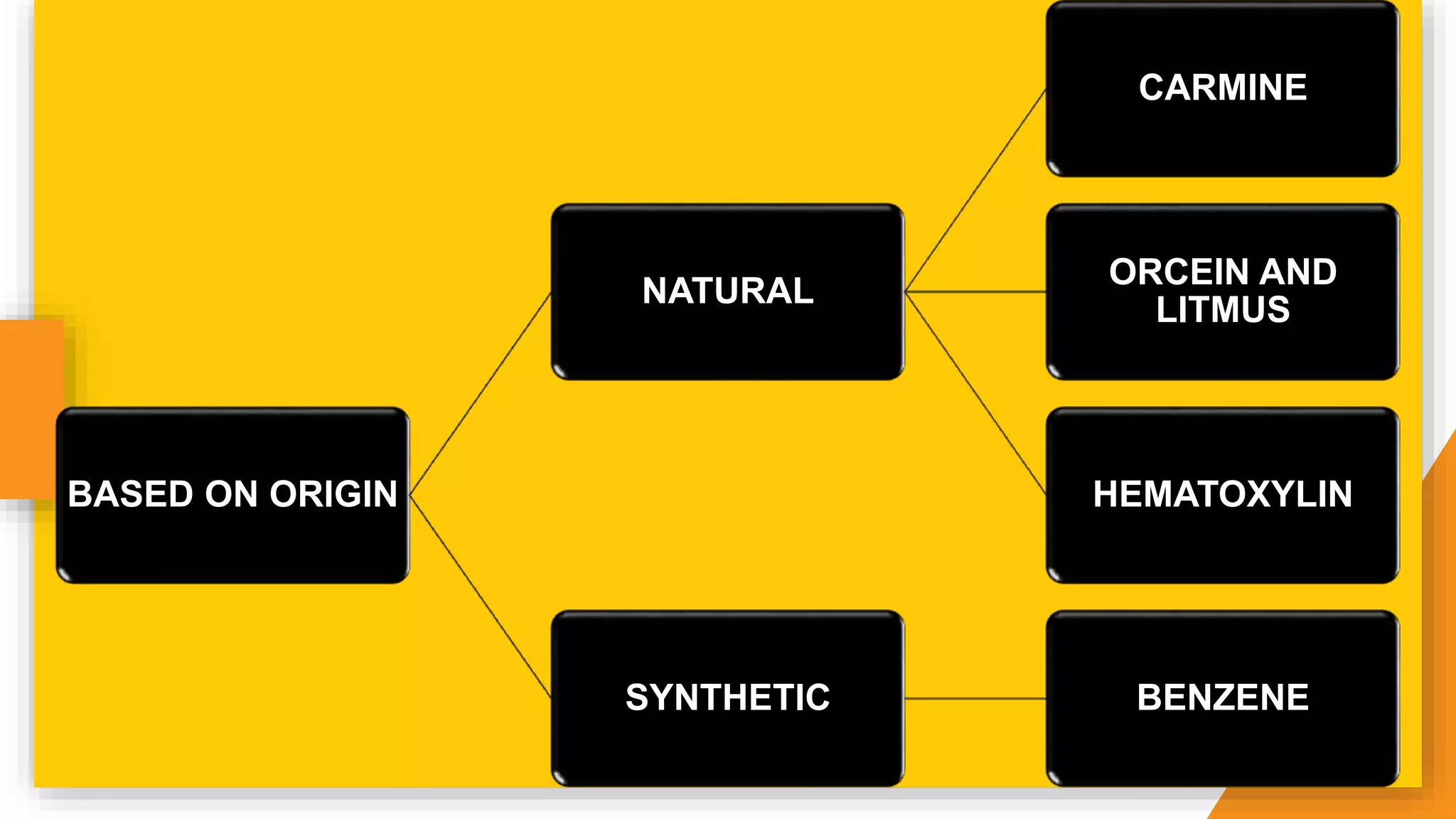
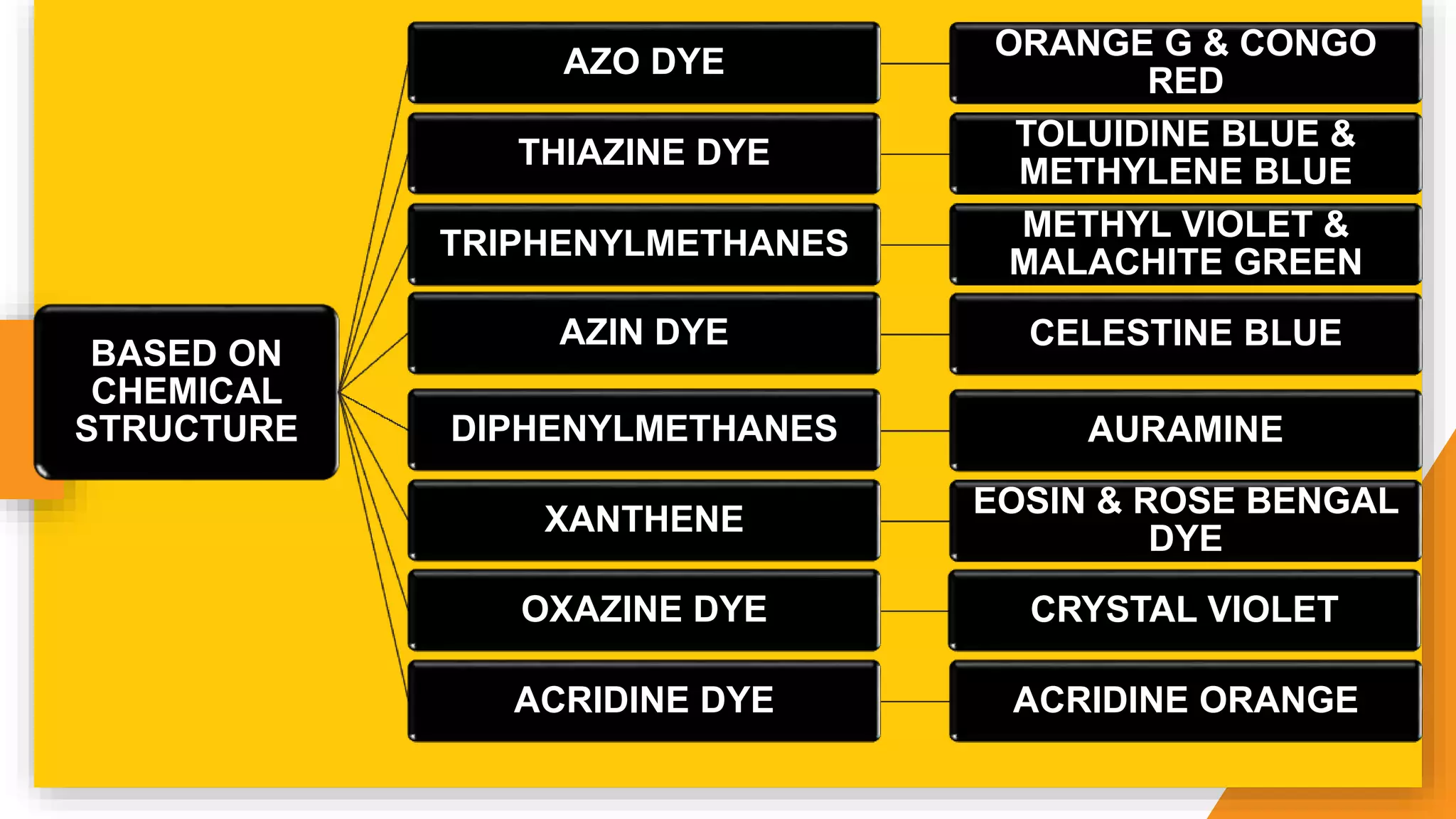
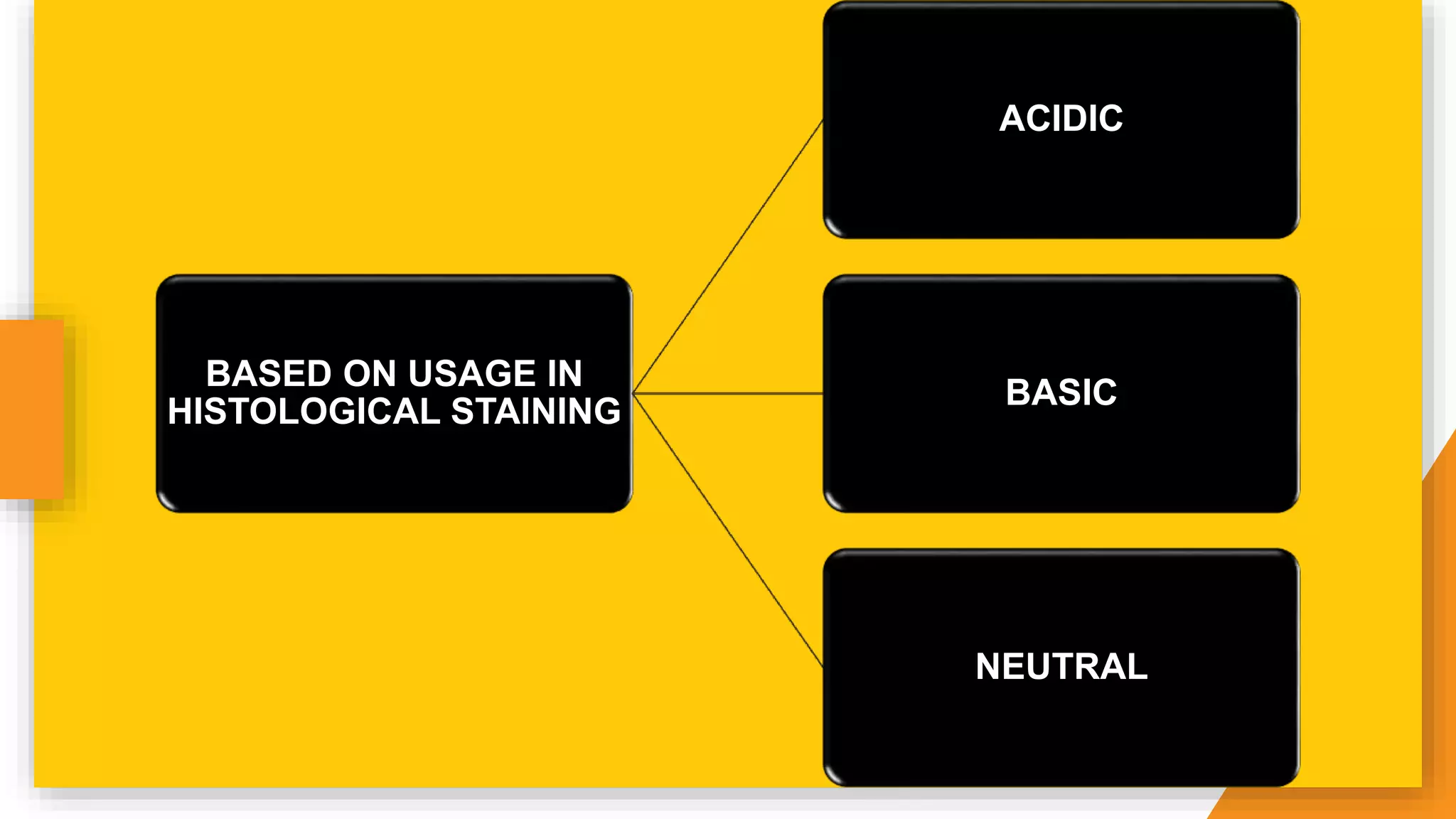
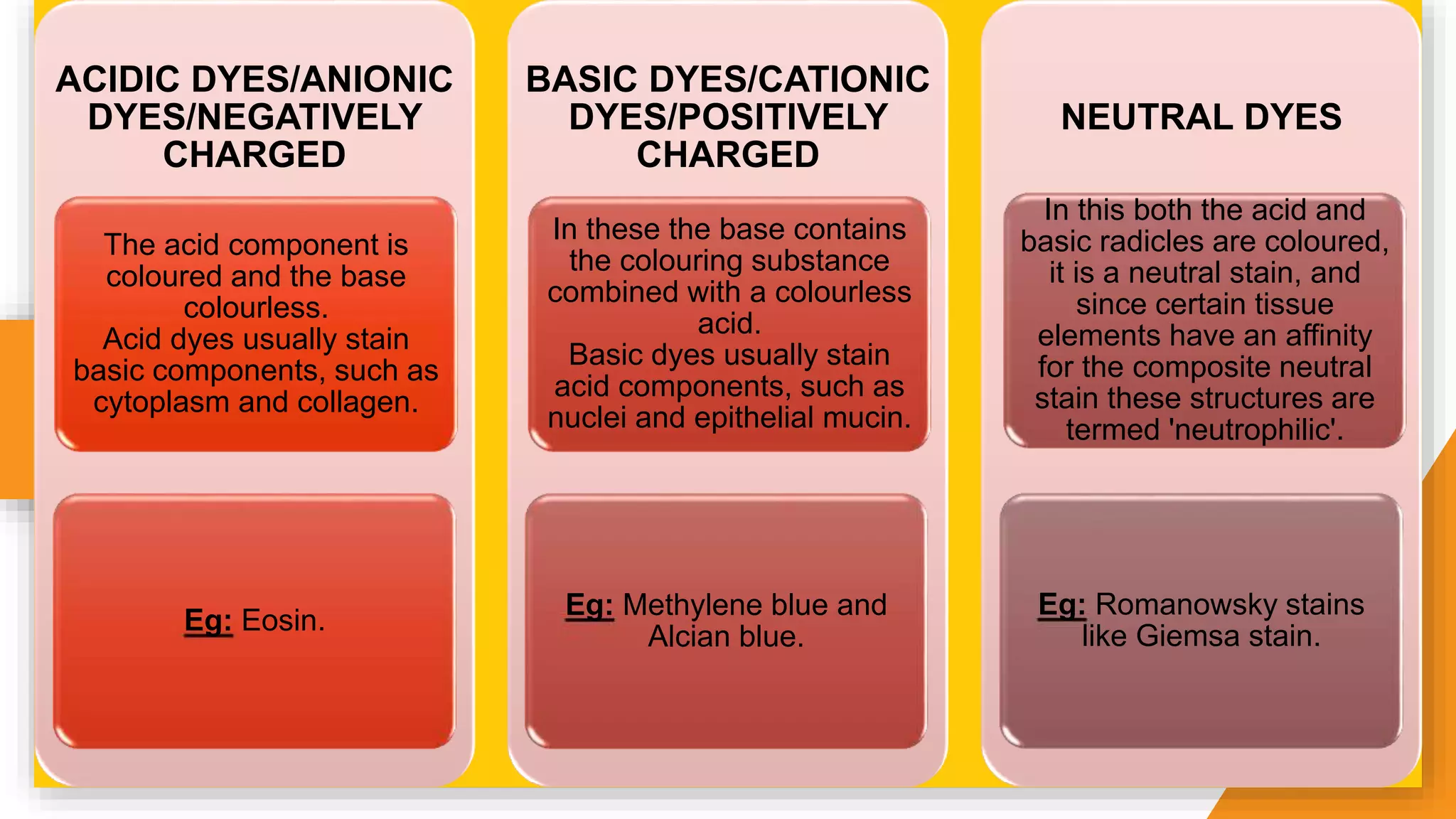
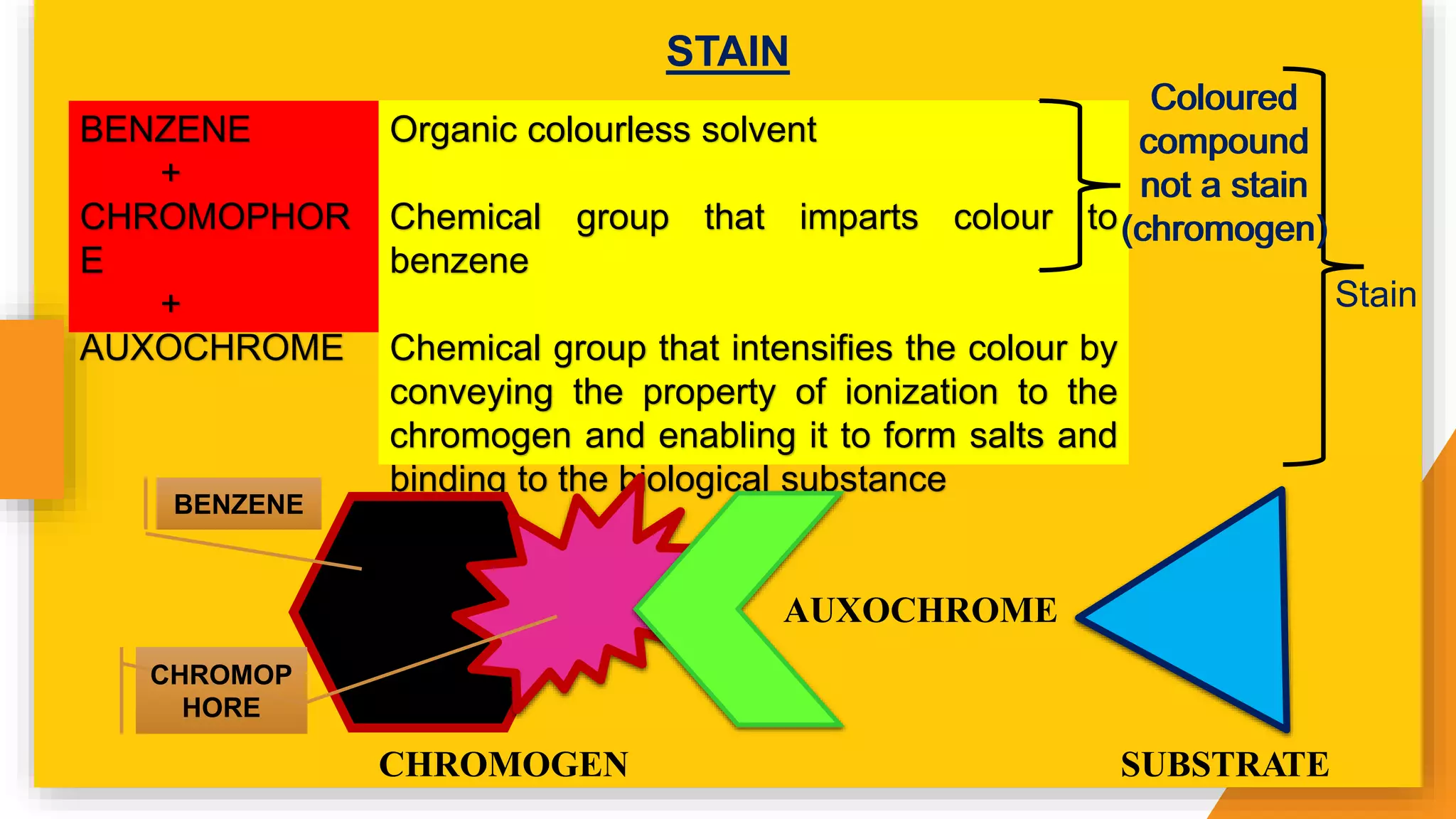
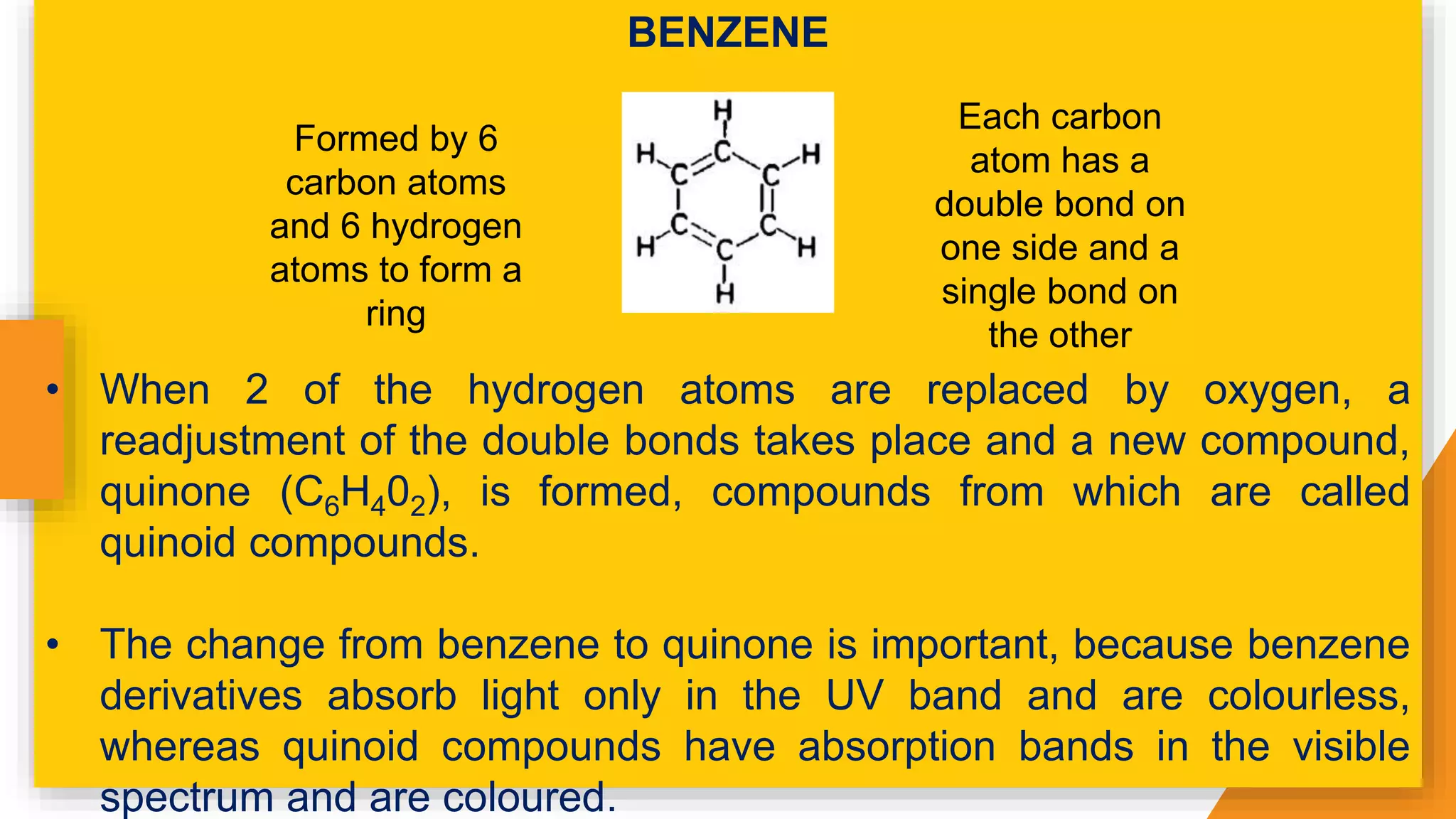
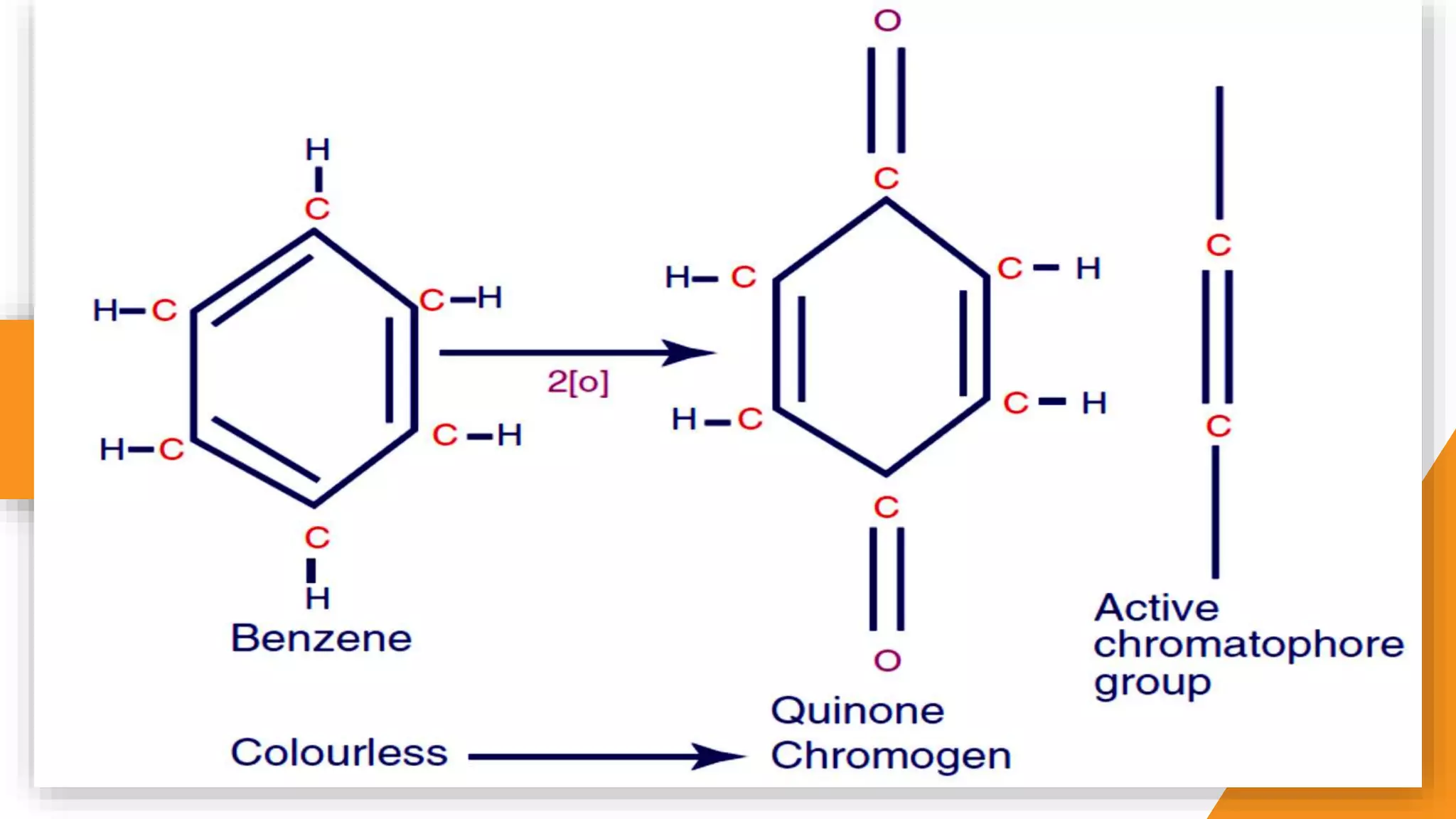
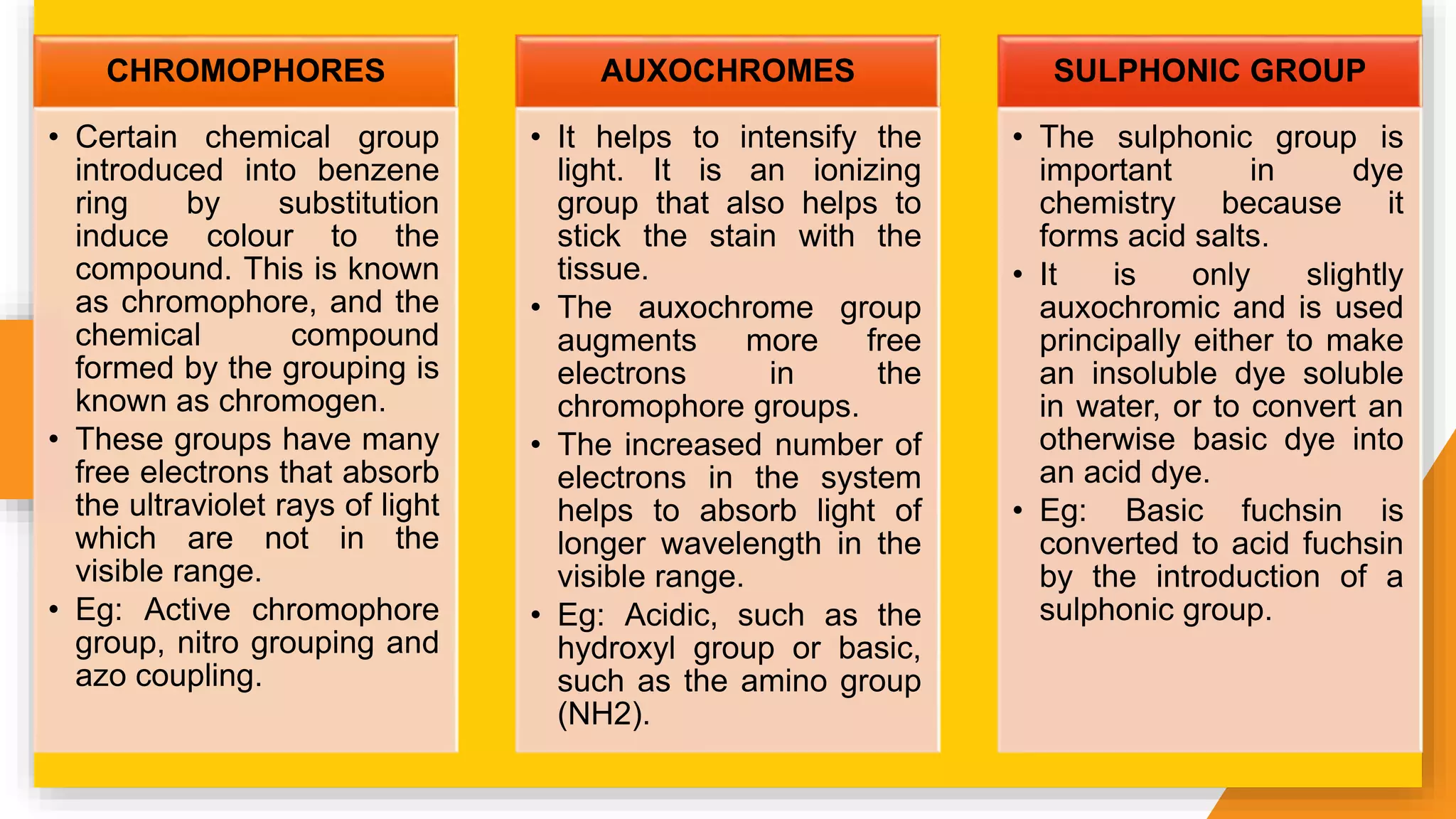
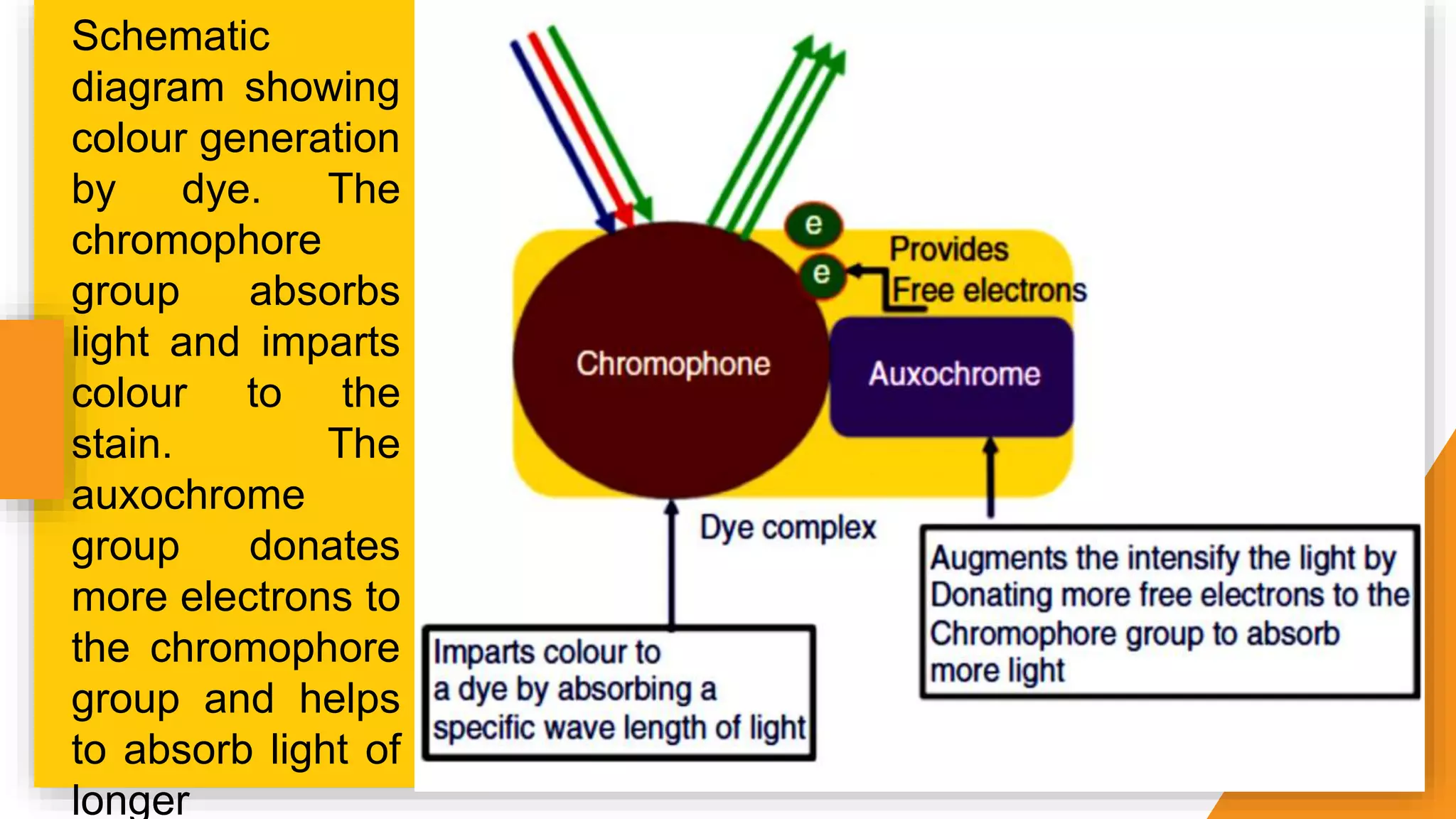
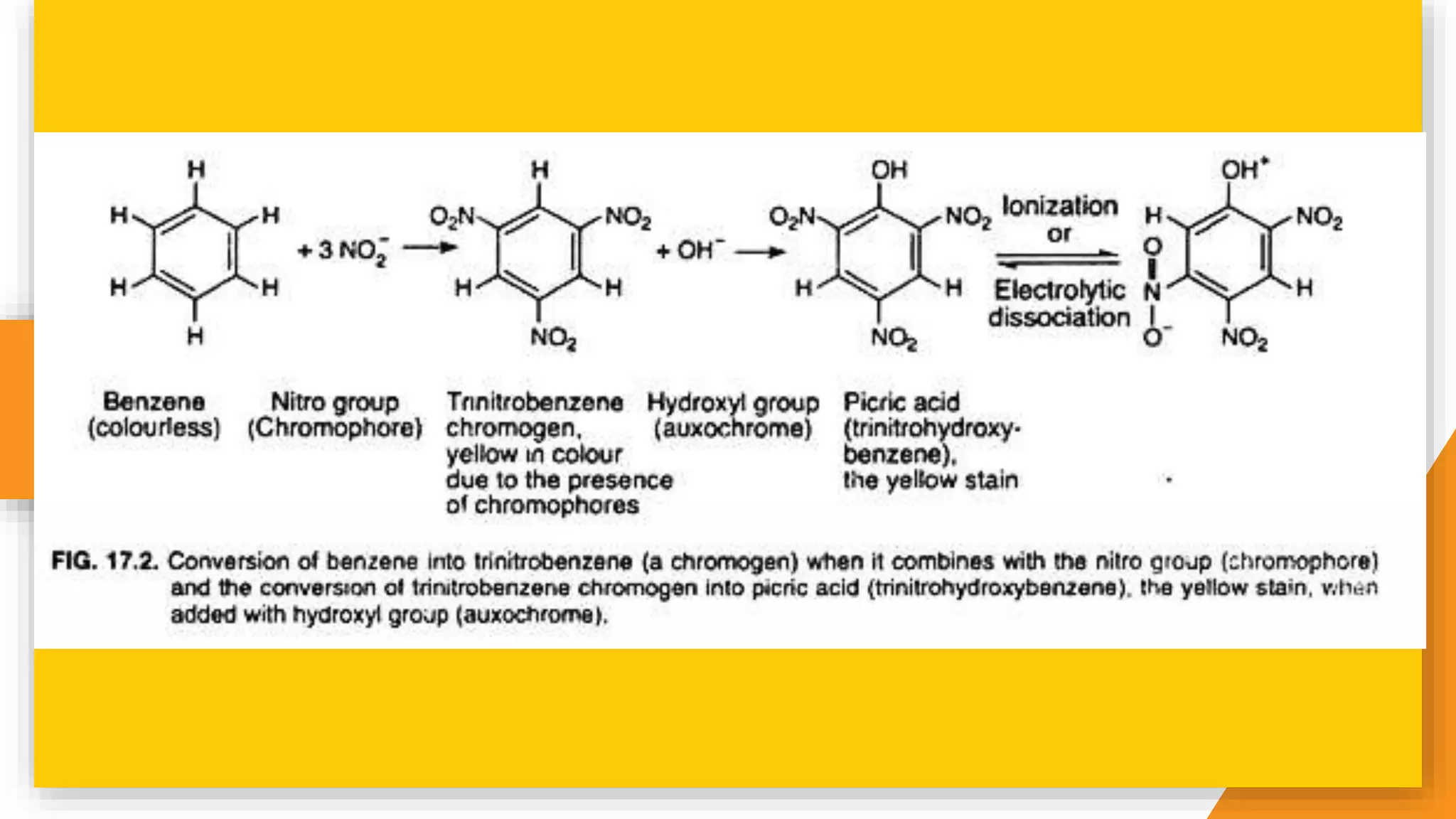
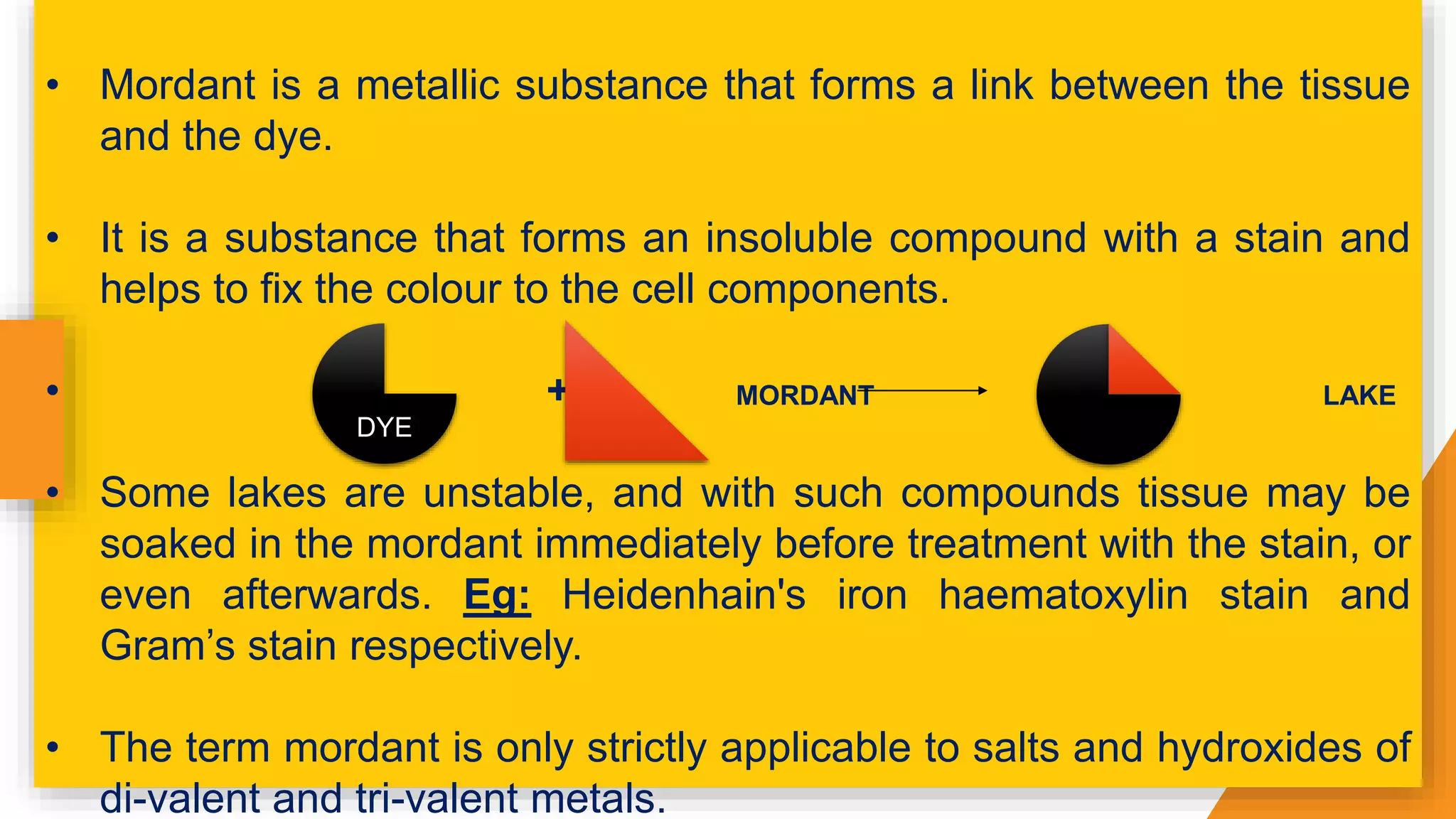
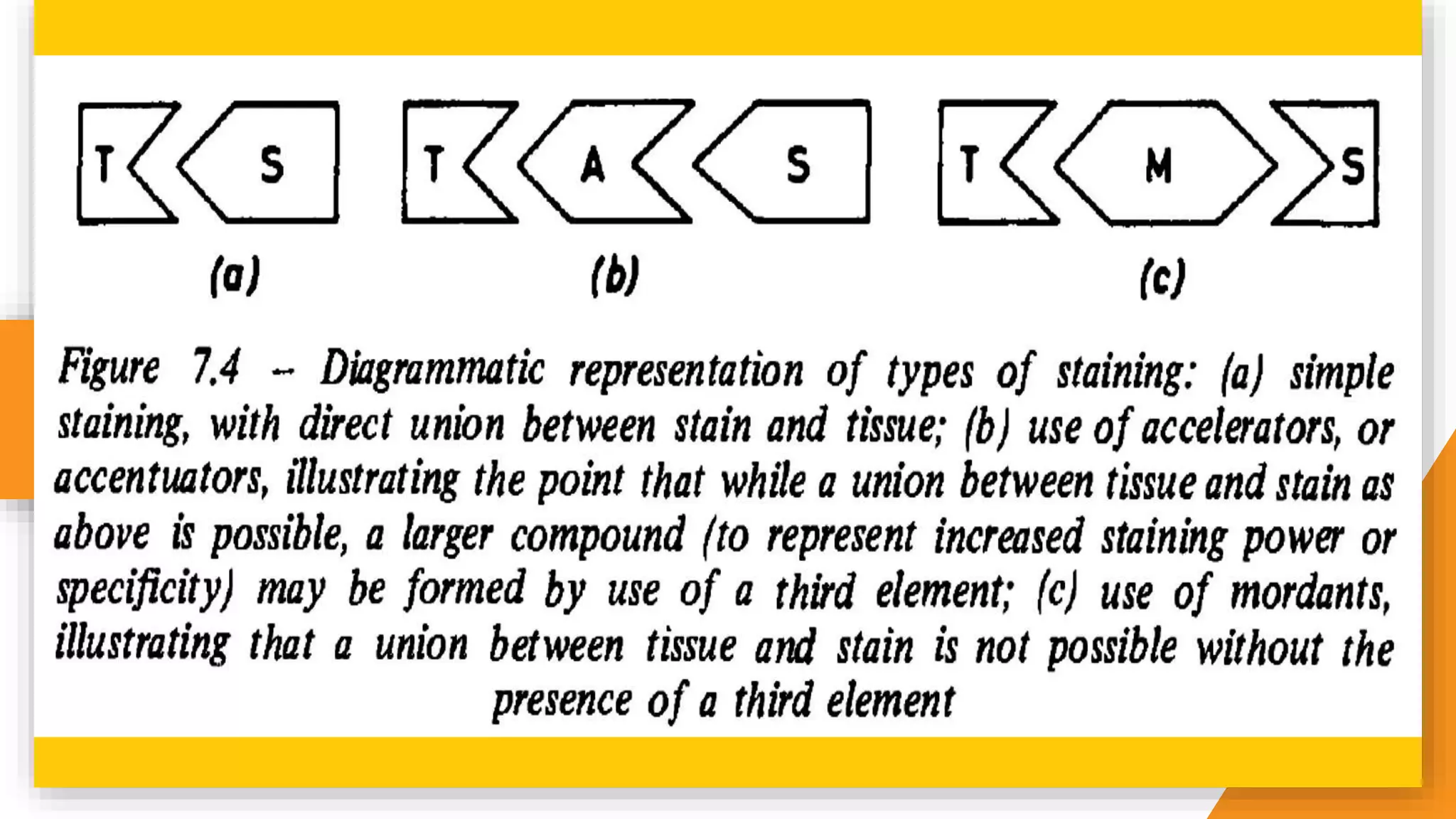
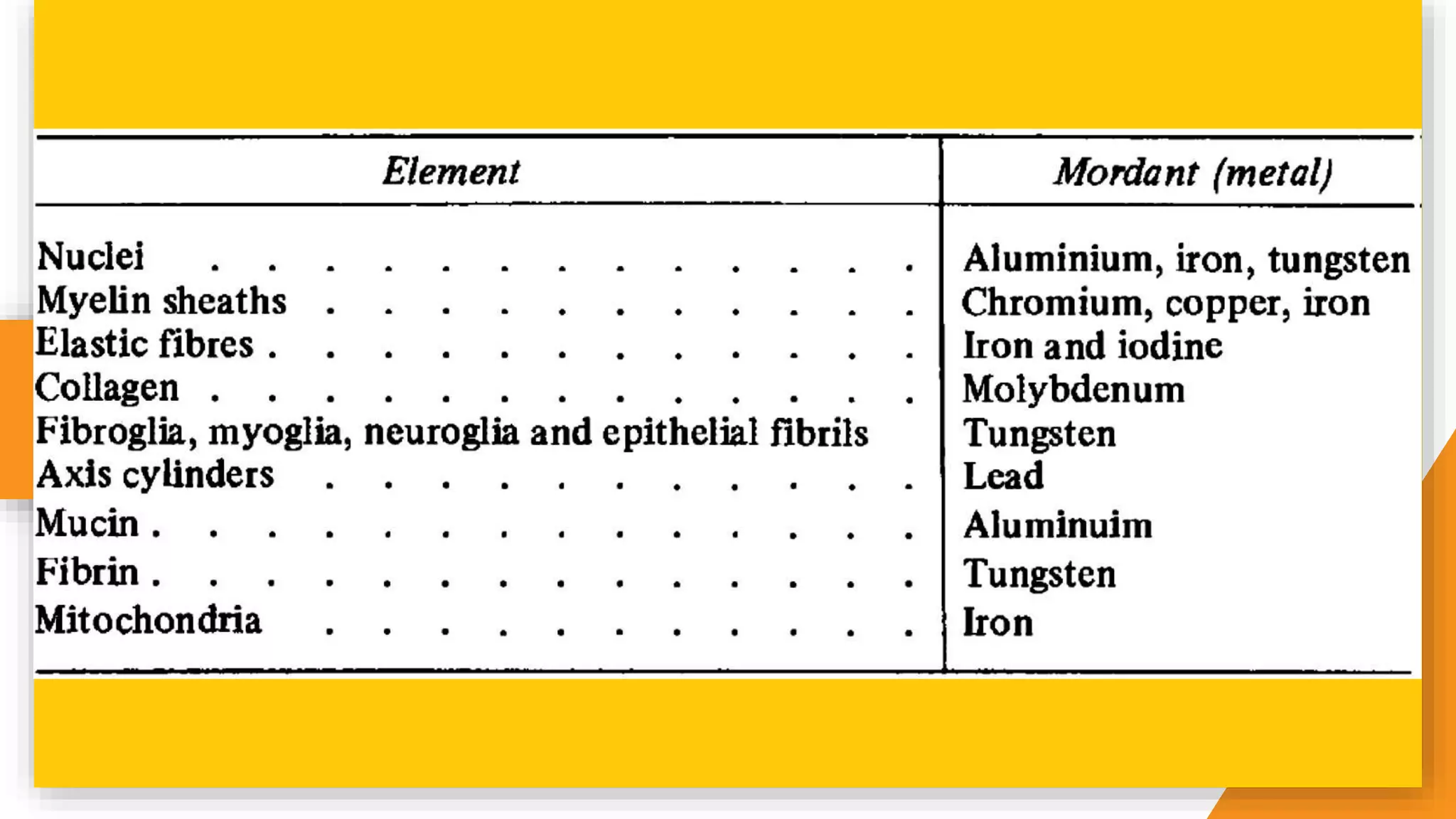
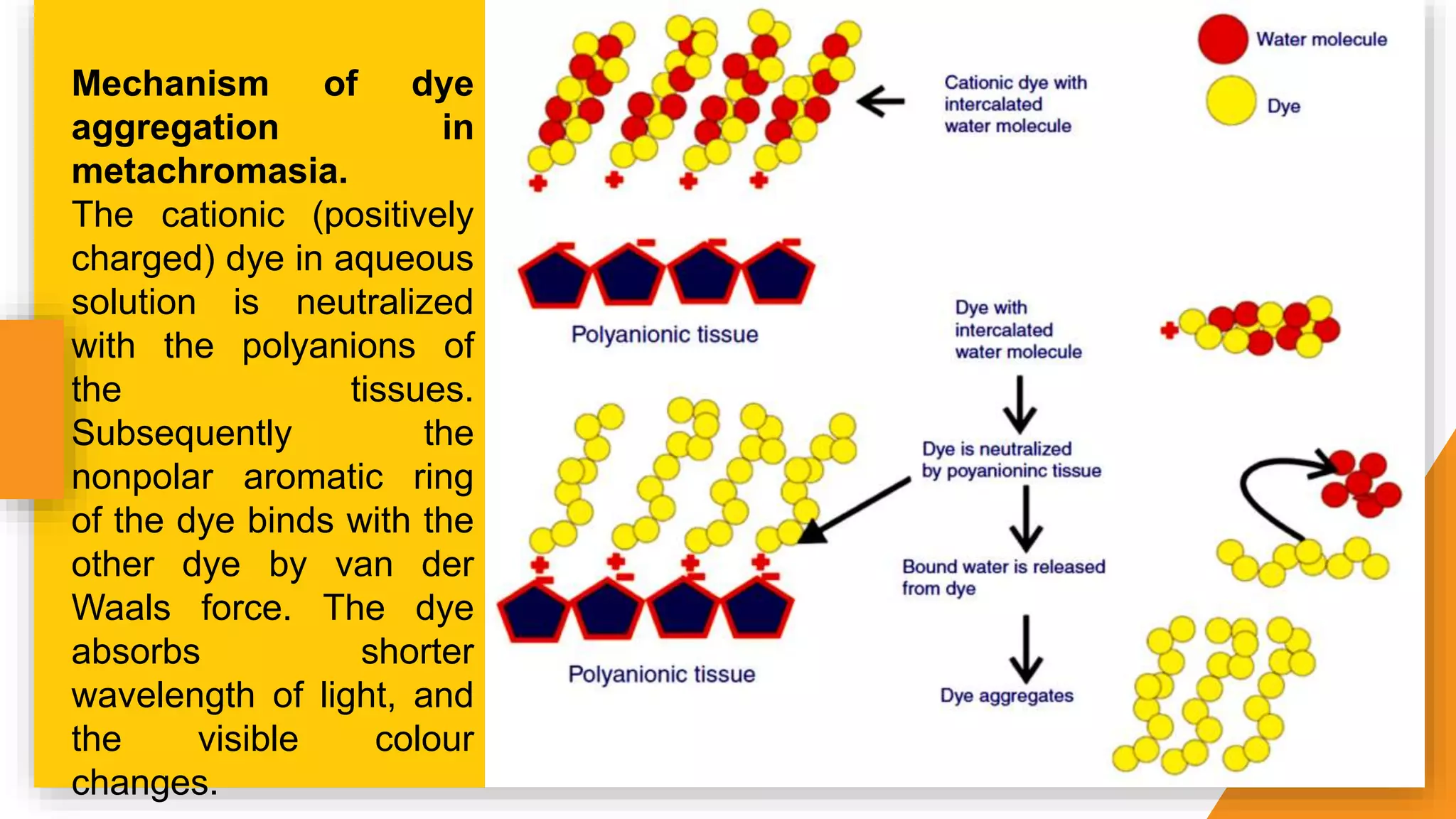
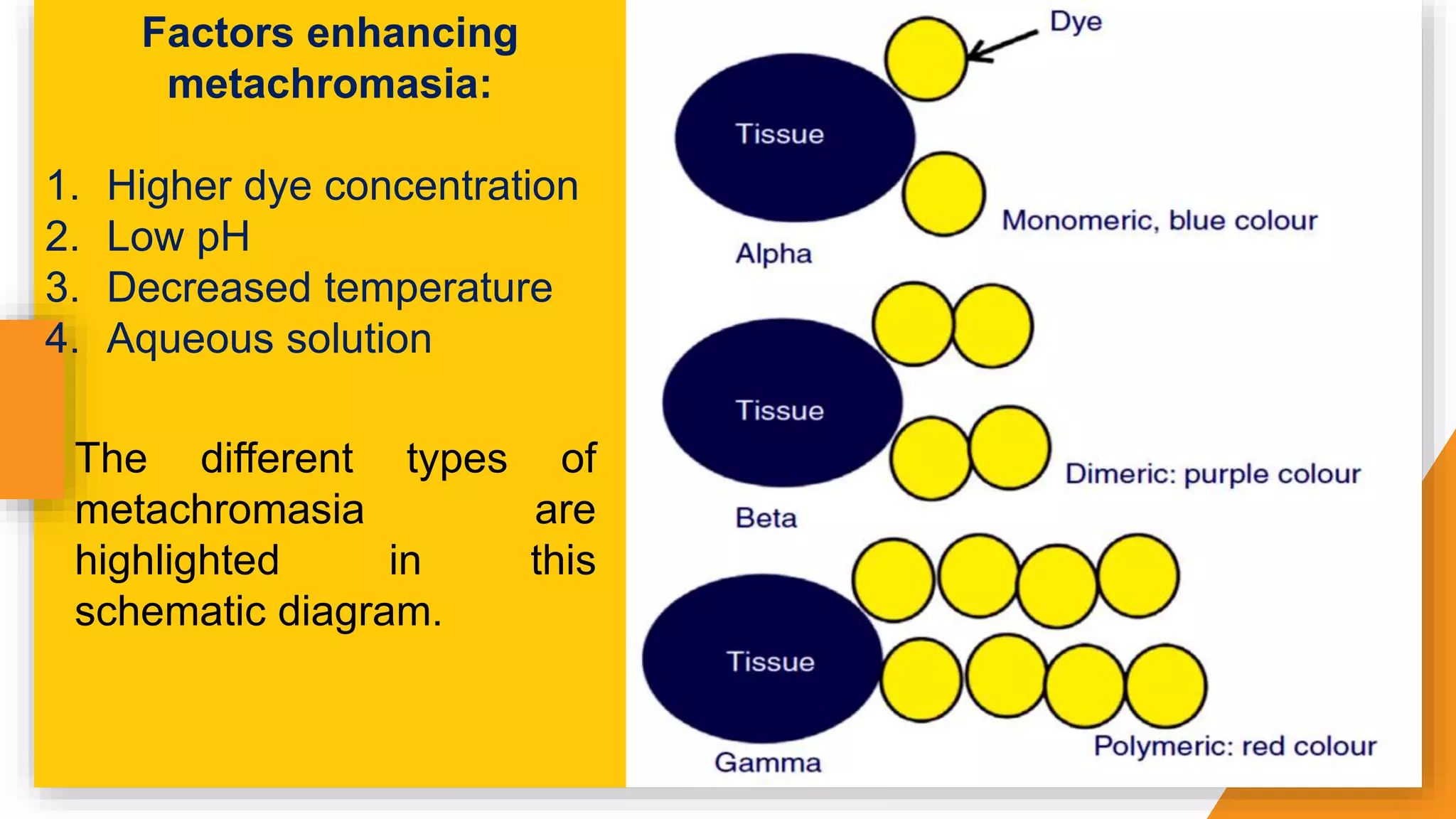
The document discusses the principles and applications of staining in biological tissues, including definitions, history, types of staining, and the chemistry behind dyes. Staining is essential for visualizing tissue components, and the proper selection of dyes is influenced by factors such as tissue affinity and dye properties. Various staining techniques, including direct and indirect staining, as well as the use of mordants and accentuators, are detailed alongside their effects on tissue visualization.