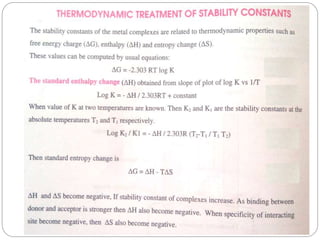
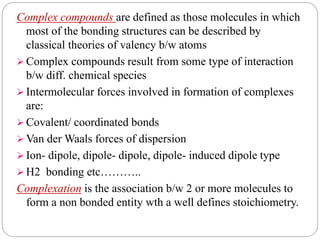
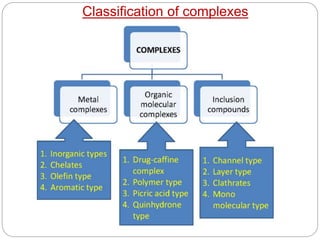
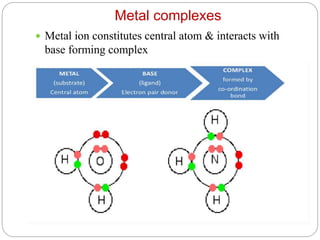
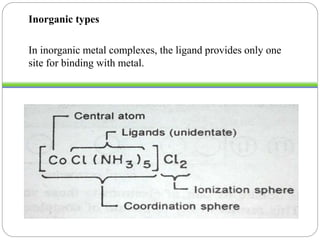
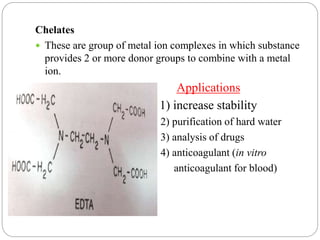
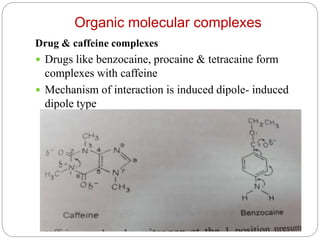
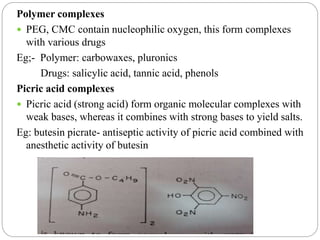
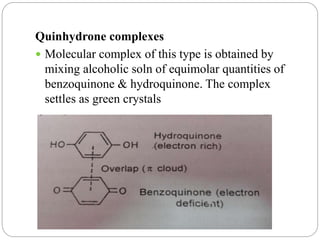
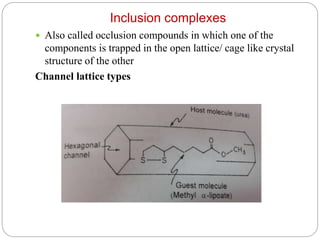
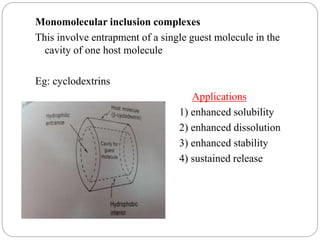
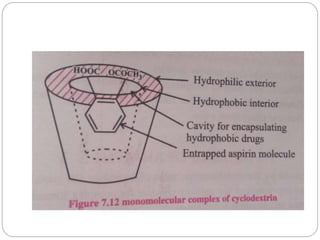
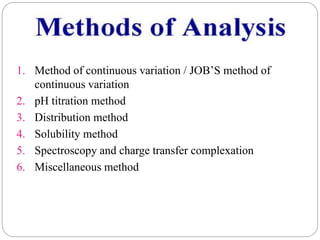
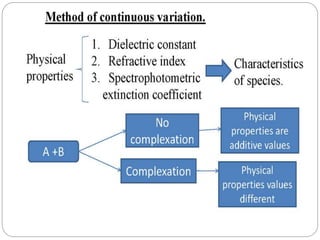
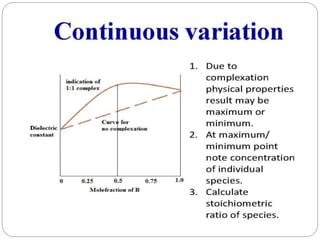
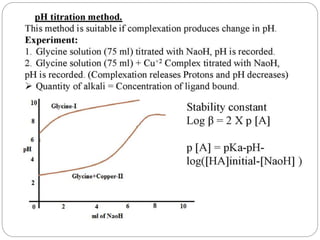
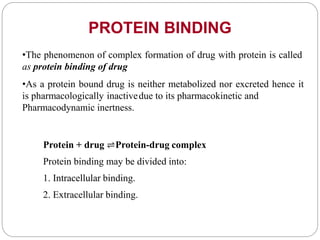
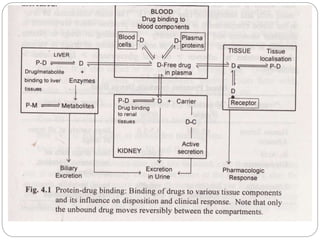
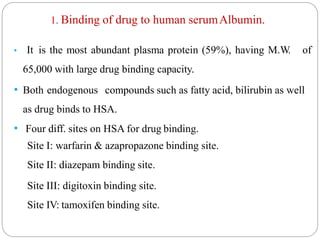
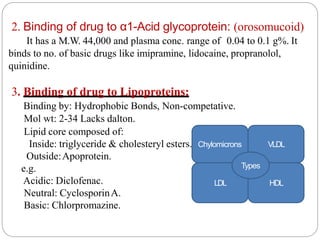
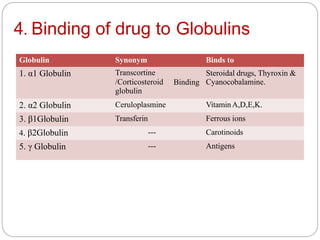
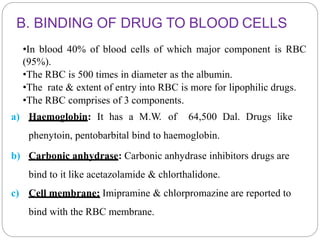
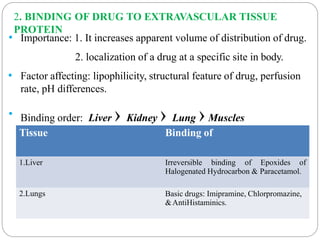
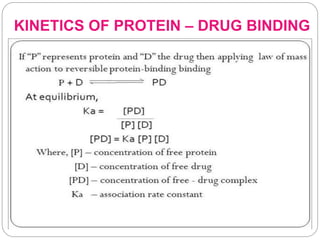
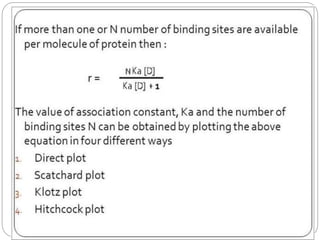
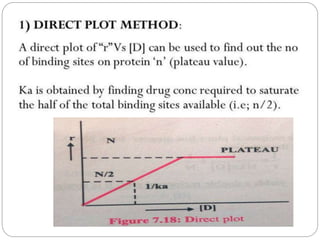
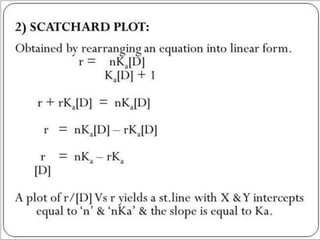
Complex compounds are formed from interactions between different chemical species and have various applications in pharmacy, including improving drug stability, solubility, and bioavailability. The document discusses the types of complexes, such as metal complexes and organic molecular complexes, along with their uses in drug formulation and toxicity reduction. It also covers the significant role of protein-drug binding in drug distribution, metabolism, and action.















![3. Drug interactions
drugs for the binding sites[ Displacementa. Competition between
interactions]:-
D2
D1+P D2+P
D1: Displaced drug. D2: Displacer drug.
e.g. Administration of phenylbutazone to a patient on Warfarin therapy results
in Hemorrhagic reaction.
b. Competition between drug & normal body constituents:-
The free fatty acids are known to interact with a no. of drugs that binds
primarily to HSA. the free fatty acid level increase in physiological, pathological
condition.](https://image.slidesharecdn.com/complexationproteinbinding-200826081419/85/Complexation-protein-binding-49-320.jpg)




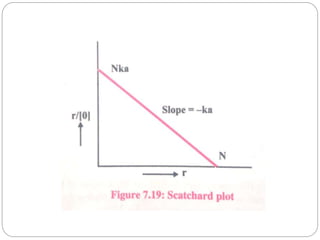
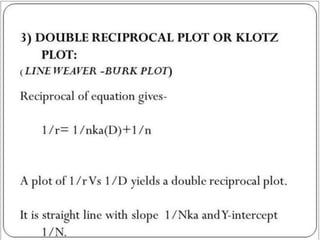
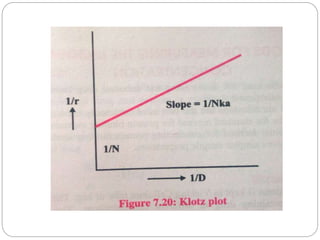
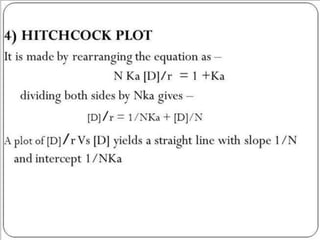

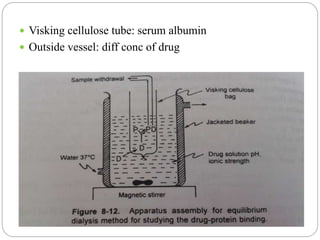
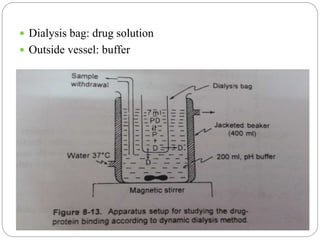
![2. Dynamic dialysis method
Its an economical method
Quick method to establish drug- protein binding
This method is based on the rate of disappearance
of drug from dialysis bag which is proportional to
conc of unbound drug
Dialysis process follows rate law,
d[Dt]/dt = k[Df]
[Dt] ------ conc of total drug
[Df] ------ conc of free(unbound) drug in bag
k --------- first order rate constant/ apparent
permeability rate constant](https://image.slidesharecdn.com/complexationproteinbinding-200826081419/85/Complexation-protein-binding-70-320.jpg)