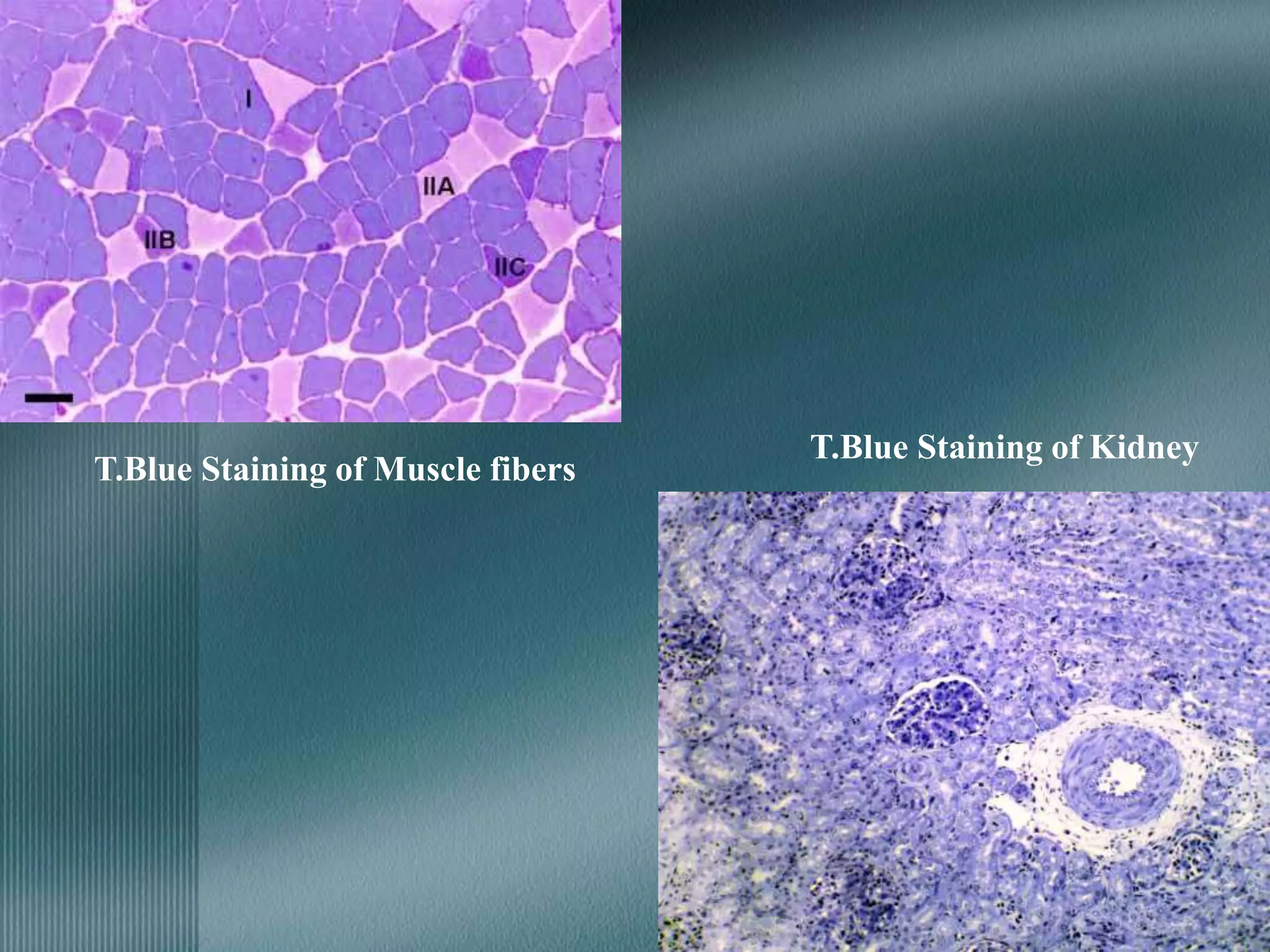
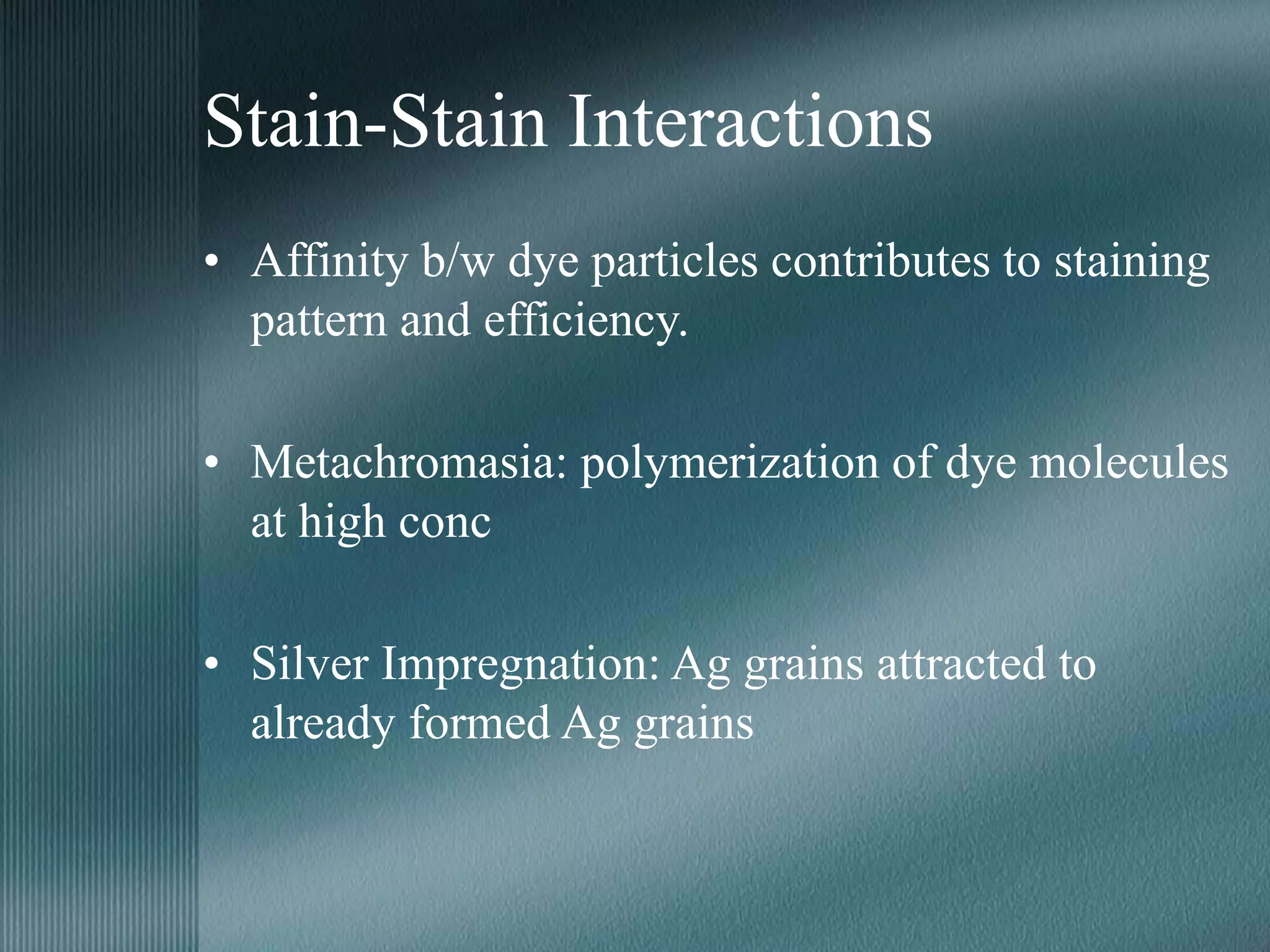
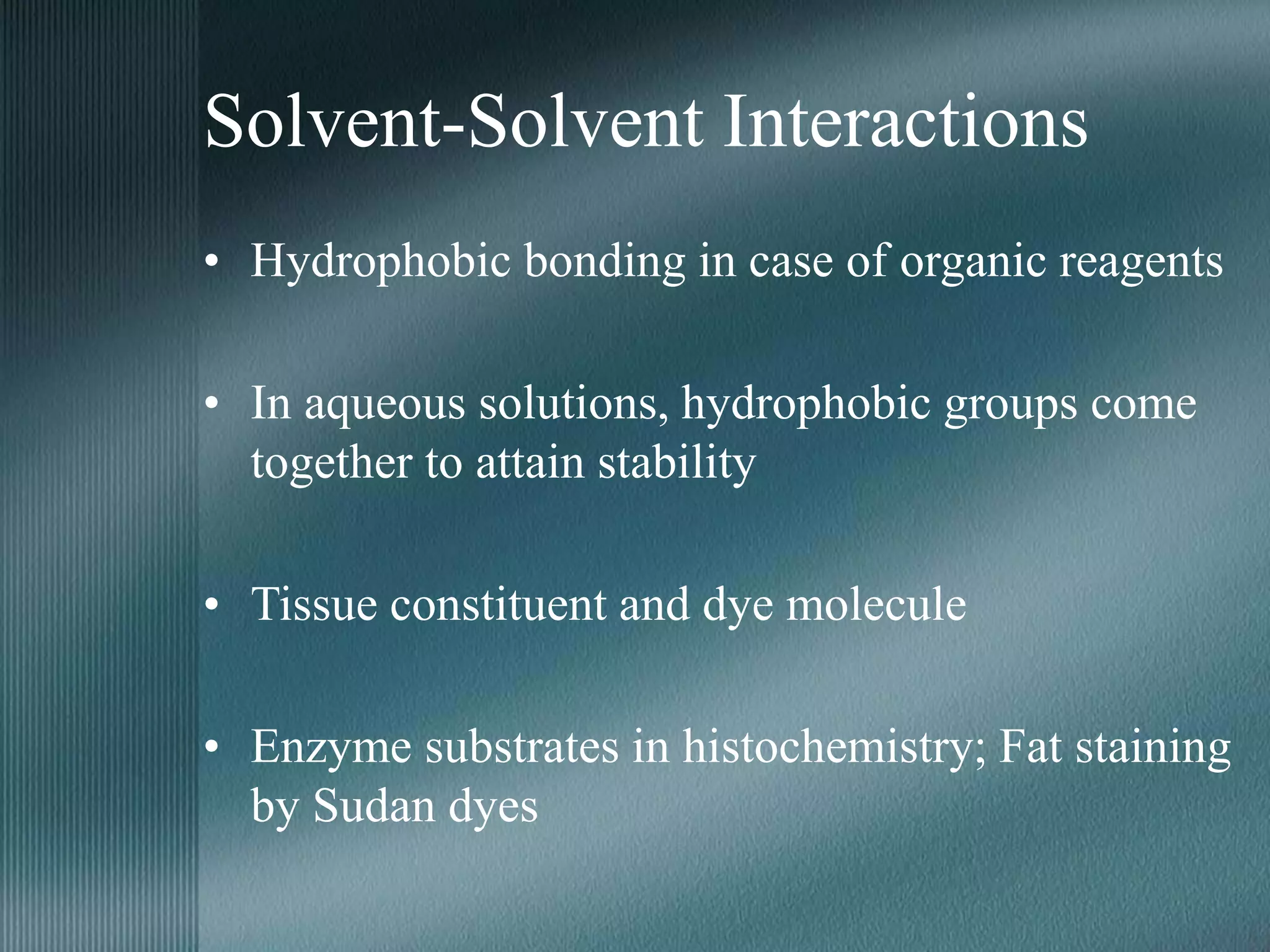
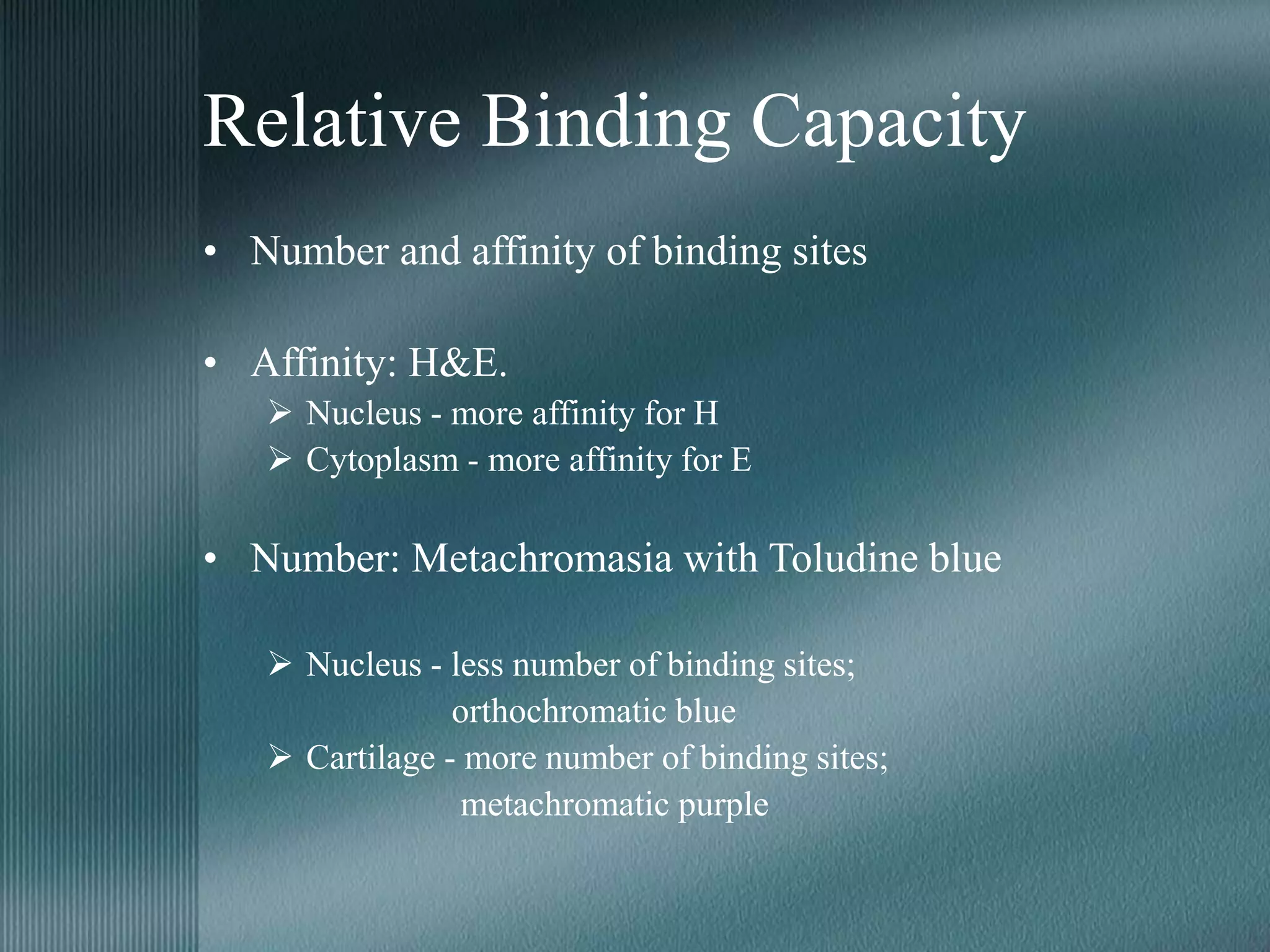
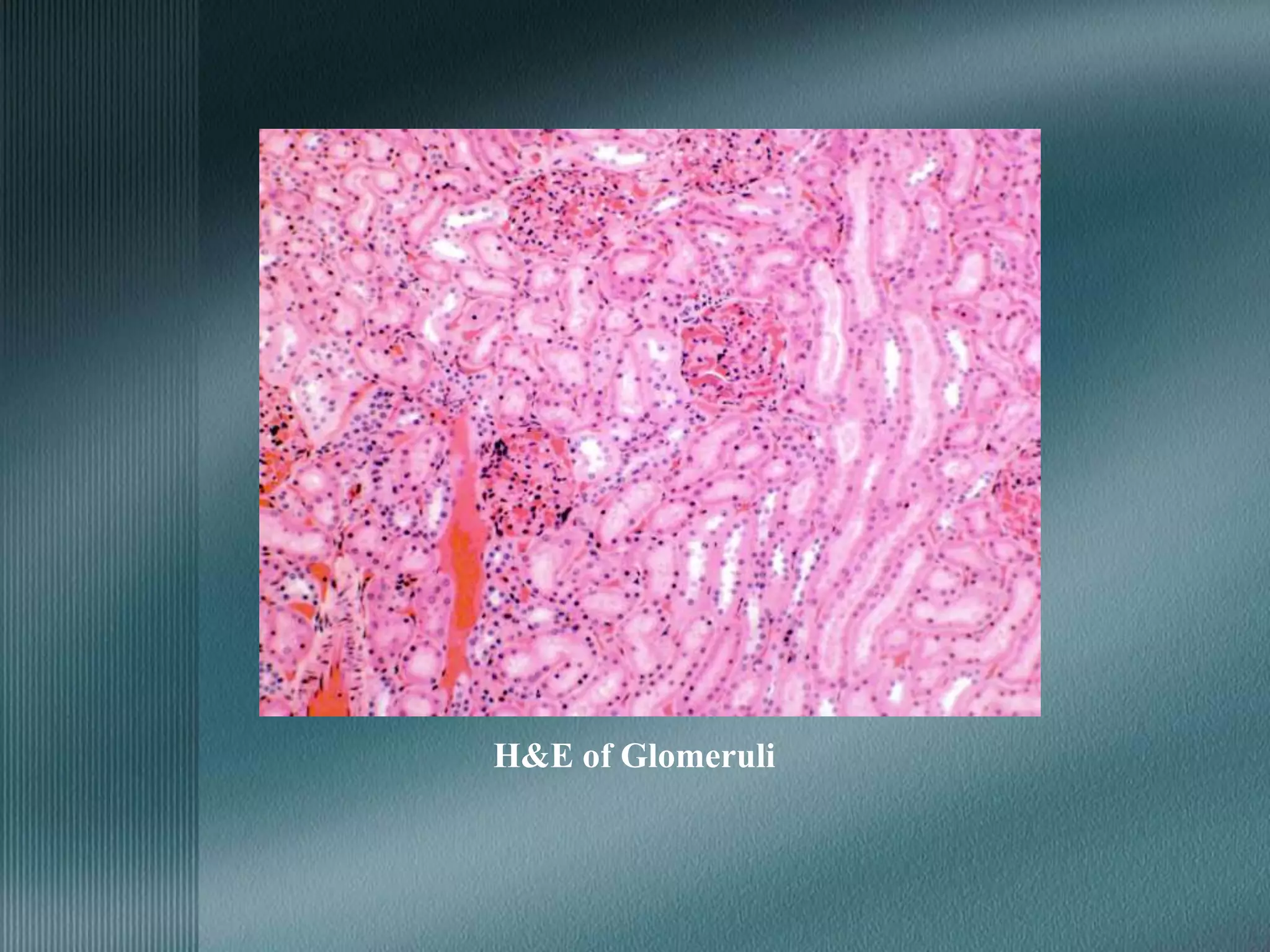
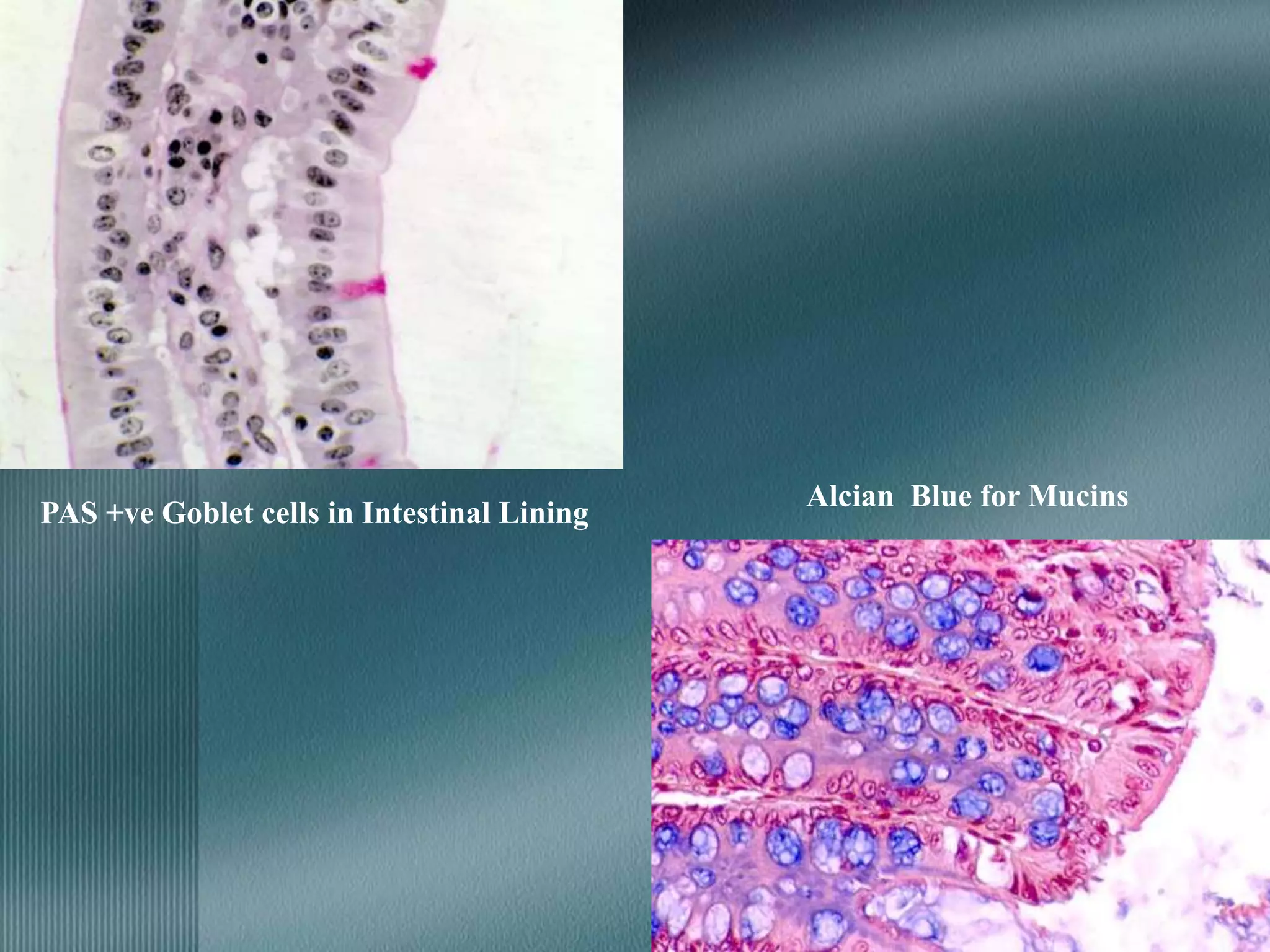
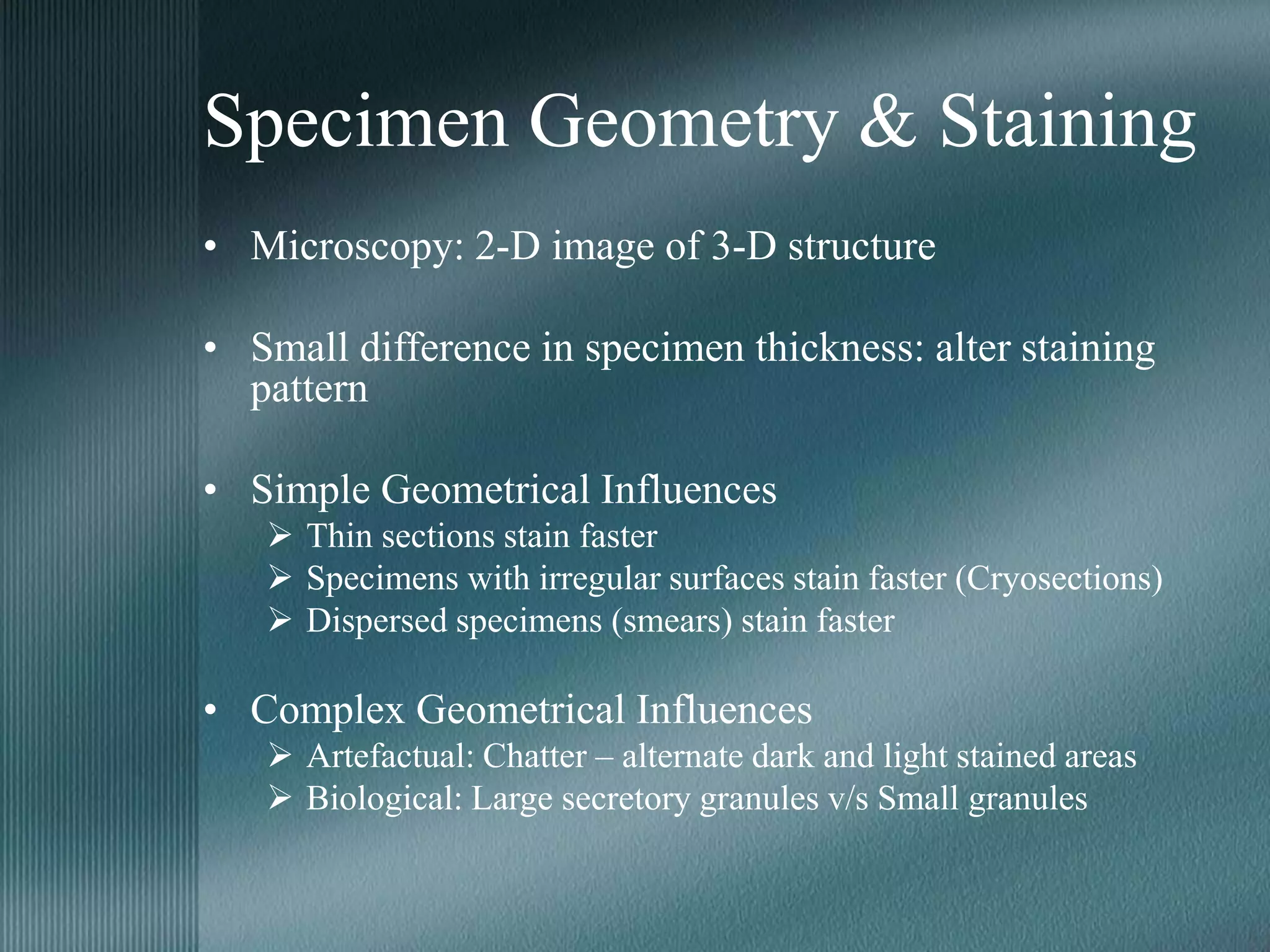
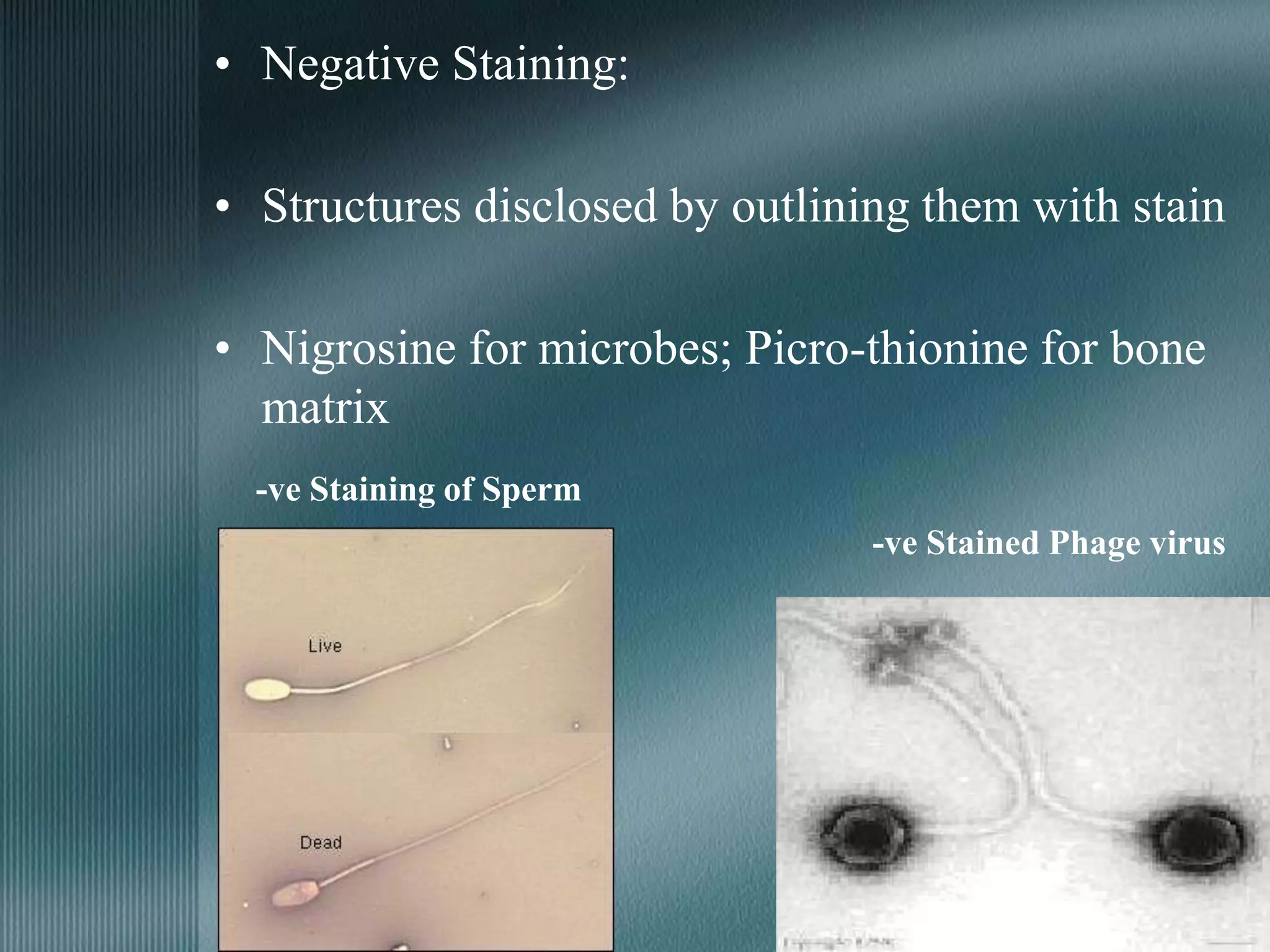
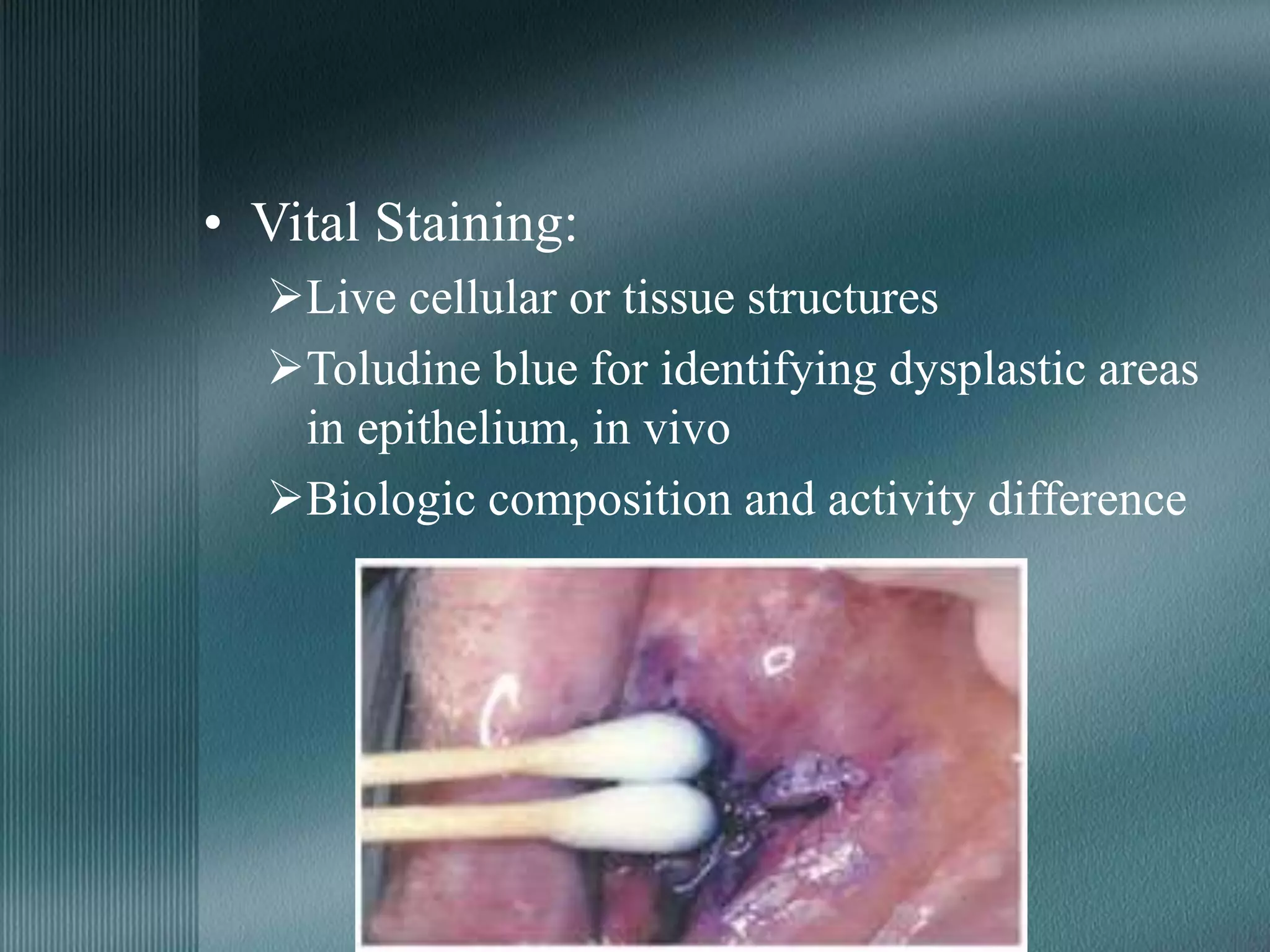
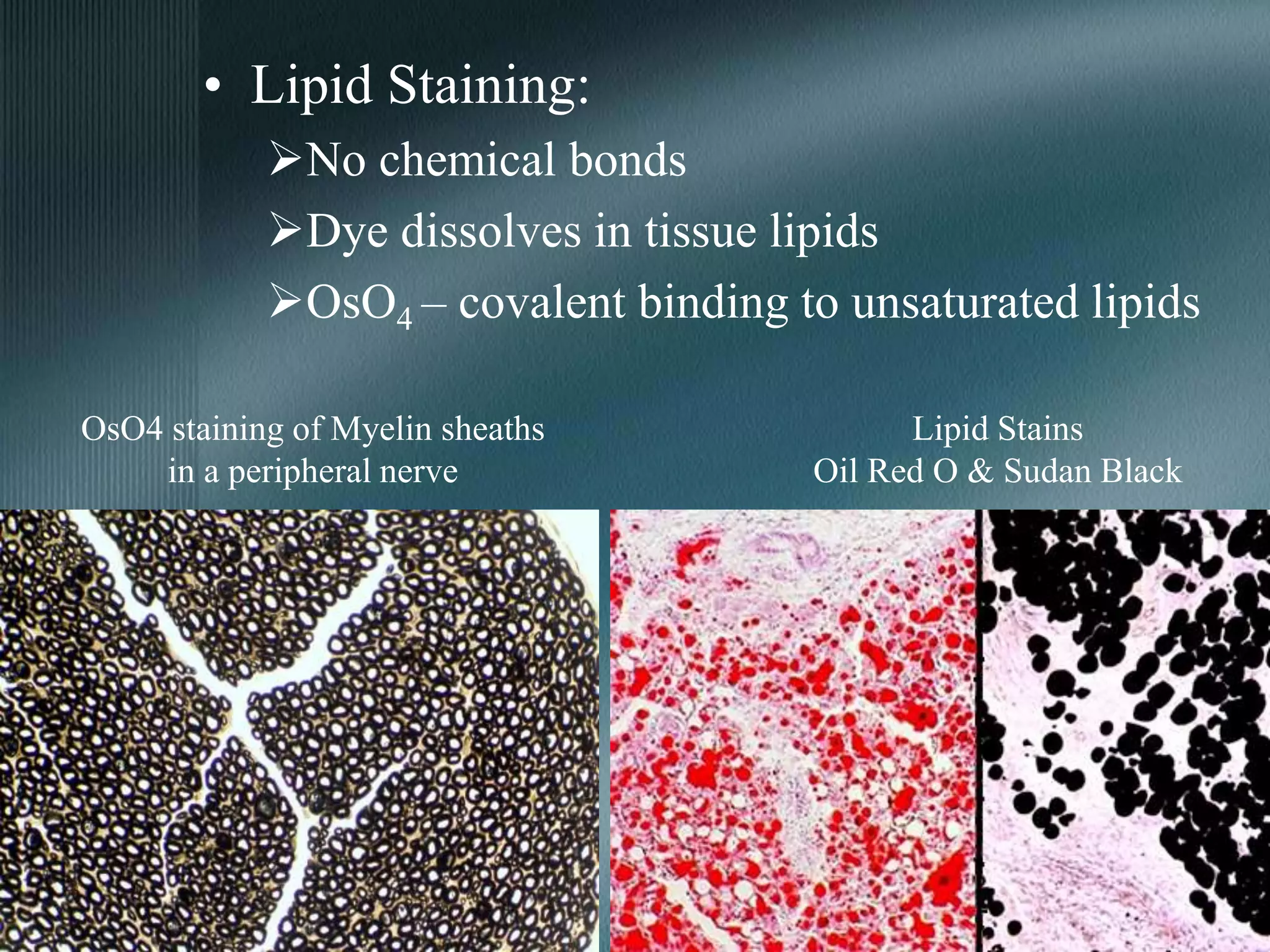
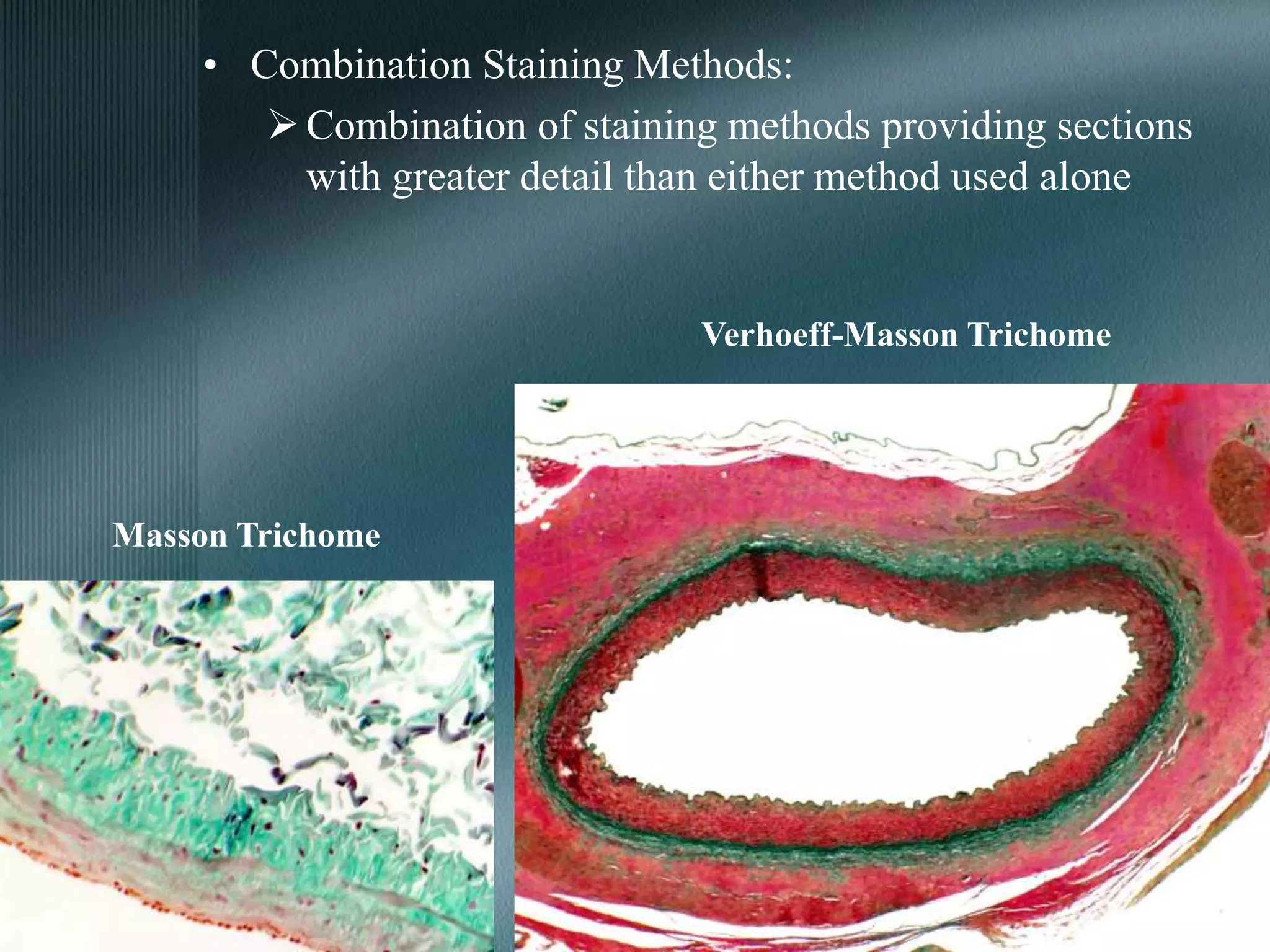
The document discusses the theory of staining in histology. It explains that staining allows tissues to be distinguished by providing different colors. Staining works through interactions between dyes and tissue components based on factors like charge, size, and chemical bonds. Differential staining is achieved through differences in binding capacity, rate, and strength between dyes and tissues. The document outlines various dye properties, classification systems, and staining methods. It also addresses factors influencing staining quality and provides examples of common problems and their solutions.