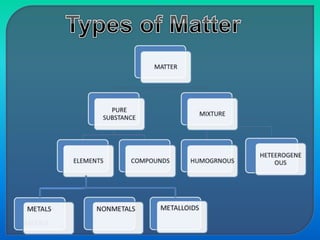
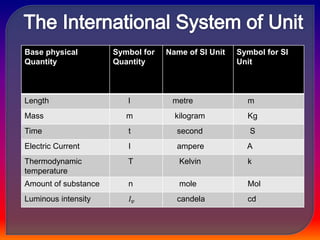
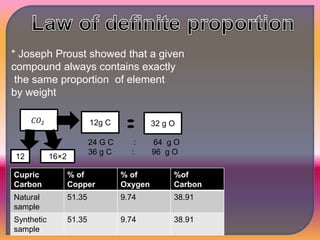
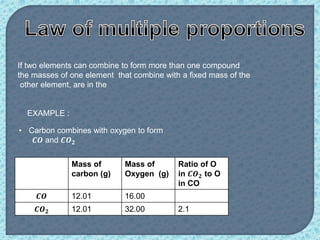
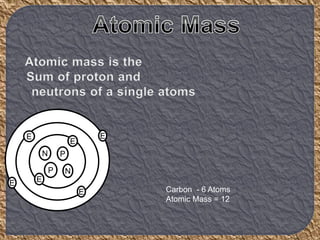
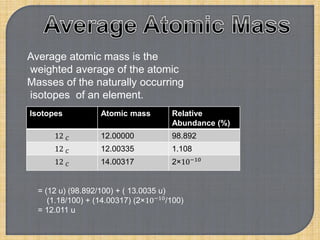
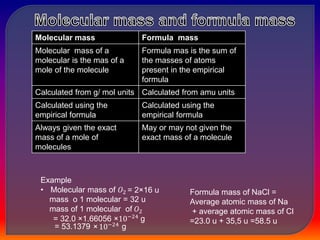
Chemistry is the study of matter, its physical and chemical properties, and the changes it undergoes. There are five basic laws that govern all chemical reactions: conservation of mass, definite proportions, multiple proportions, Gay-Lussac's law of gaseous volumes, and Avogadro's law. According to Dalton's atomic theory, atoms are indivisible and atoms of the same element have identical properties, while compounds form when atoms combine in fixed ratios in reactions where atoms are neither created nor destroyed. The mole concept allows chemists to relate the mass of a substance to the number of particles or entities it contains.