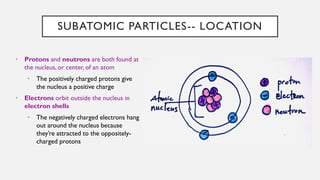
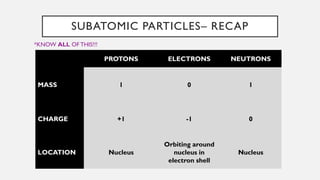
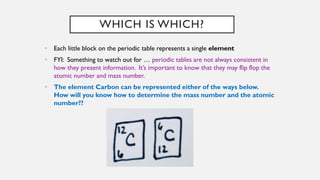
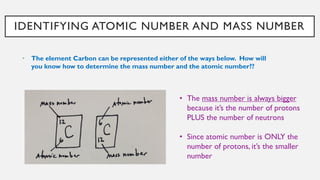
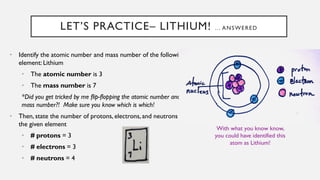
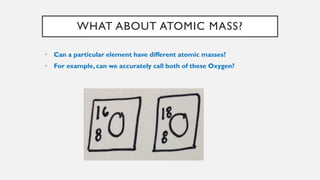
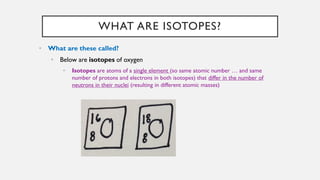
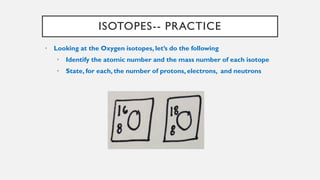
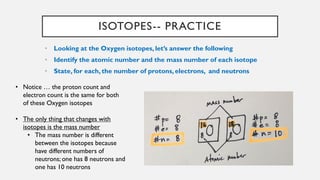
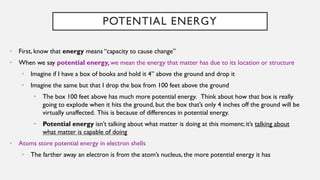
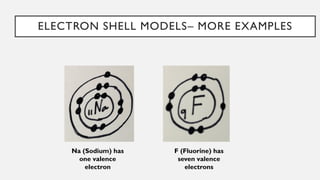
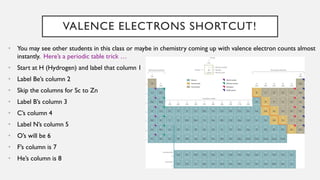
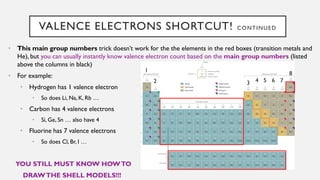
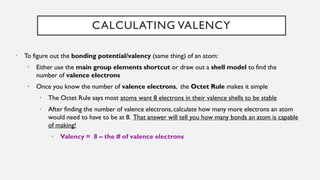
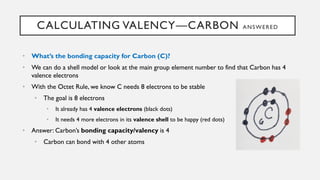
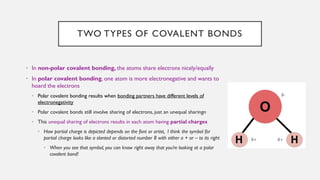
Chapter 2 discusses the essential concepts of chemistry relevant to biology, focusing on matter, elements, and atoms. Key topics include the periodic table, essential and trace elements, atomic structure, subatomic particles, isotopes, and potential energy. Understanding these concepts lays the foundation for comprehending how atoms interact and bond to form biomolecules.