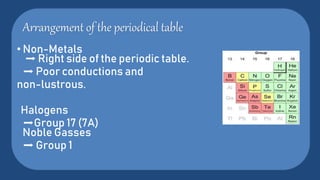
The periodic table is a systematic arrangement of all known chemical elements, organized by atomic number and electronic properties, crucial for understanding their behavior. Credited to Dmitri Mendeleev in 1869, it evolved through earlier works by scientists like Johann Döbereiner and John Newlands. The table categorizes elements into groups such as metals, nonmetals, and metalloids, helping to illustrate their chemical reactivity and properties.