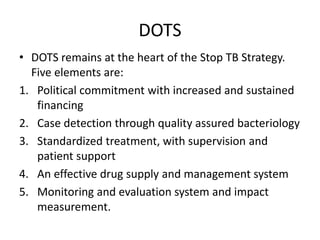
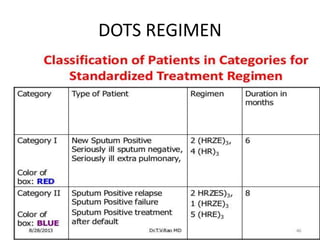
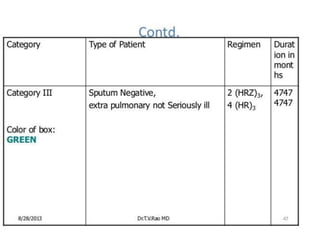
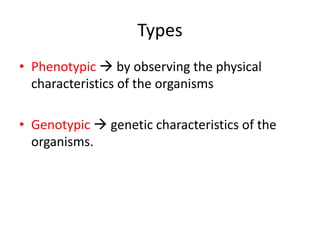
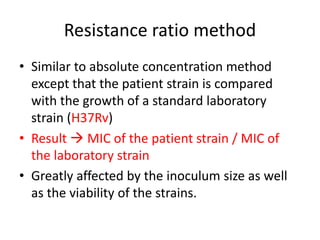
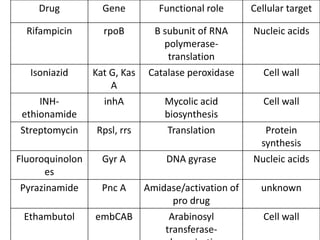
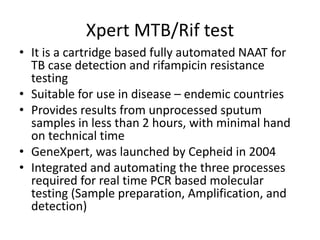
This document discusses antitubercular drugs and drug susceptibility testing. It provides details on several first and second line antitubercular drugs including isoniazid, rifampicin, streptomycin, pyrazinamide, ethambutol, thioacetazone, fluoroquinolones, macrolides, and newer drugs. It describes the DOTS regimen and criteria for drug susceptibility testing. Both phenotypic and genotypic methods for drug susceptibility testing are outlined, including the proportion method, BACTEC, MGIT, and molecular techniques like Xpert MTB/Rif.