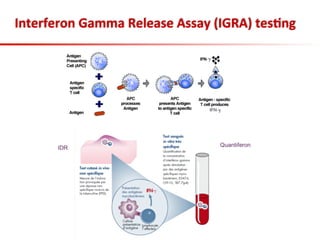
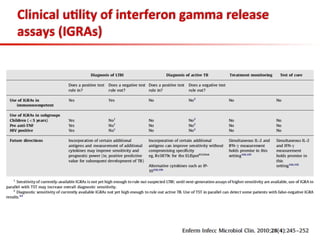
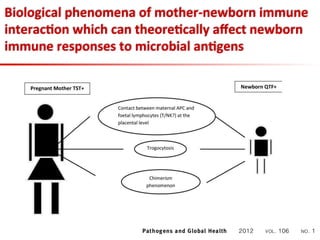
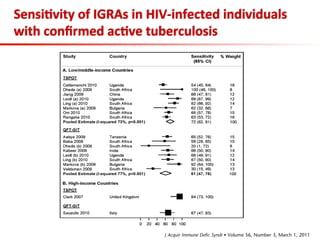
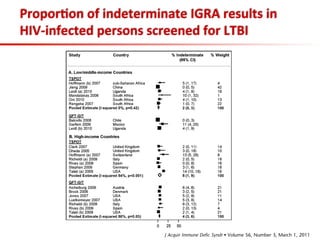
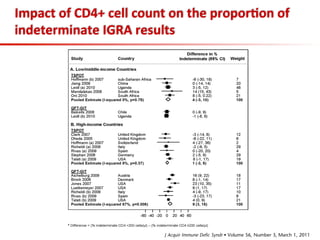
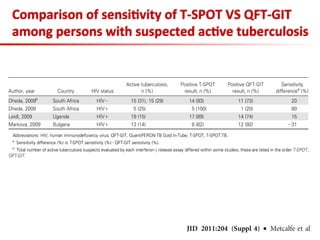
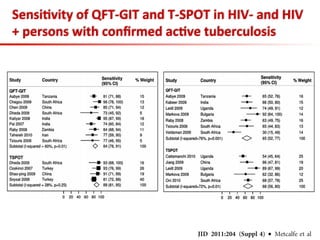
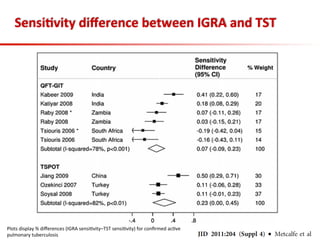
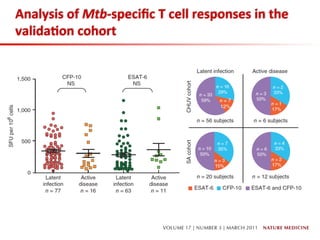
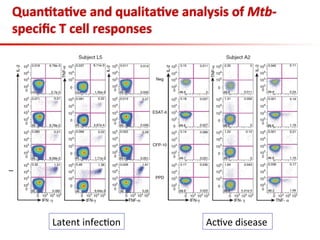
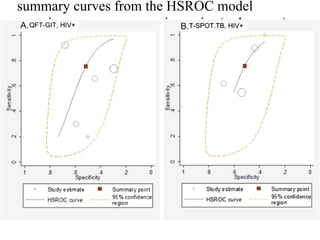
This document summarizes a presentation on immunological testing for tuberculosis (TB) and HIV co-infection. It discusses the clinical utility of interferon gamma release assays (IGRAs) for detecting latent TB infection (LTBI) in HIV-infected individuals. While IGRAs perform similarly to the tuberculin skin test (TST) in identifying those who could benefit from LTBI treatment, important questions remain about their use in HIV-positive populations with different CD4 counts. The document also examines the diagnostic value of IGRAs for active TB, finding no evidence they are more sensitive than the TST, especially in low- and middle-income countries.