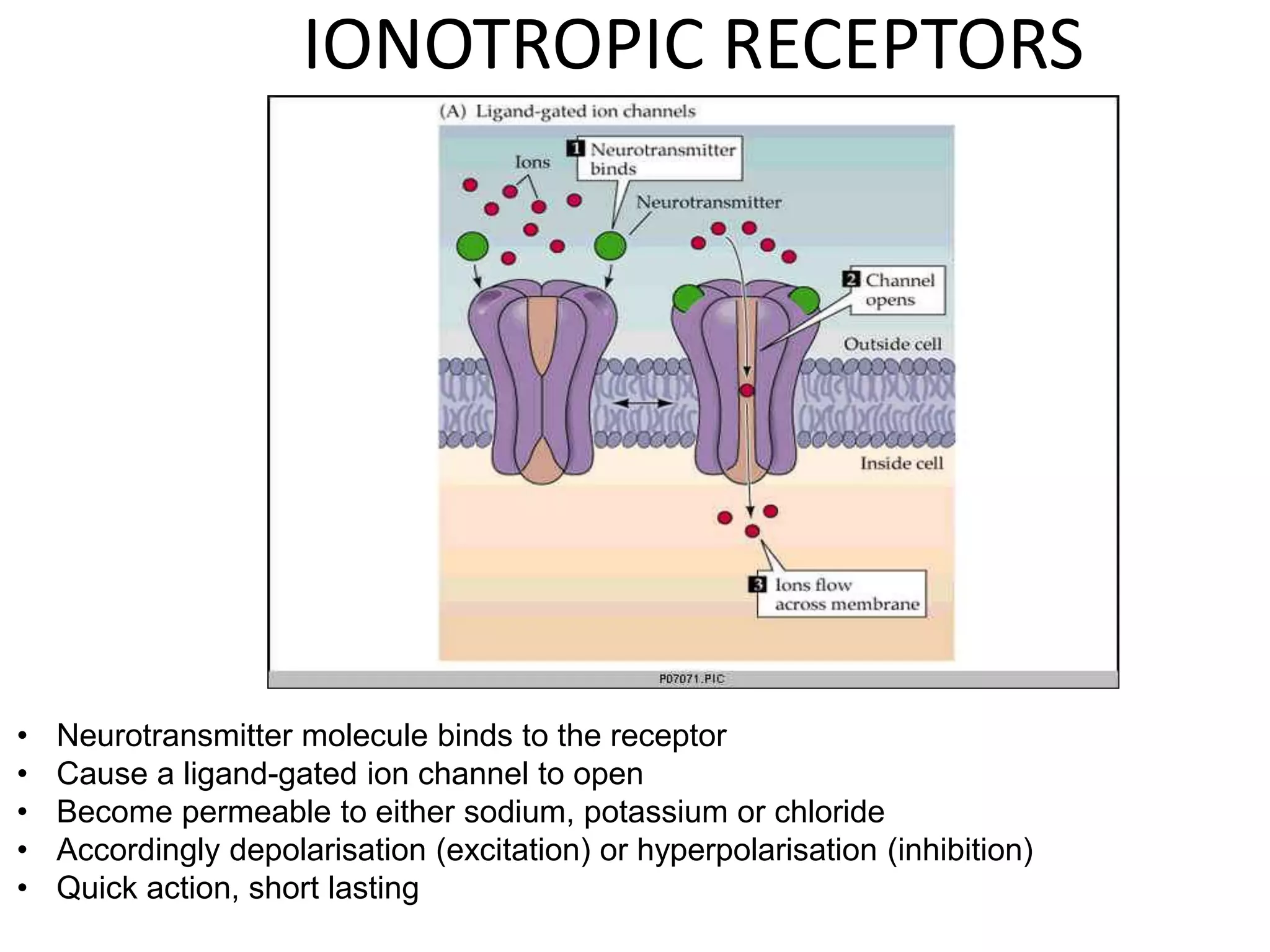
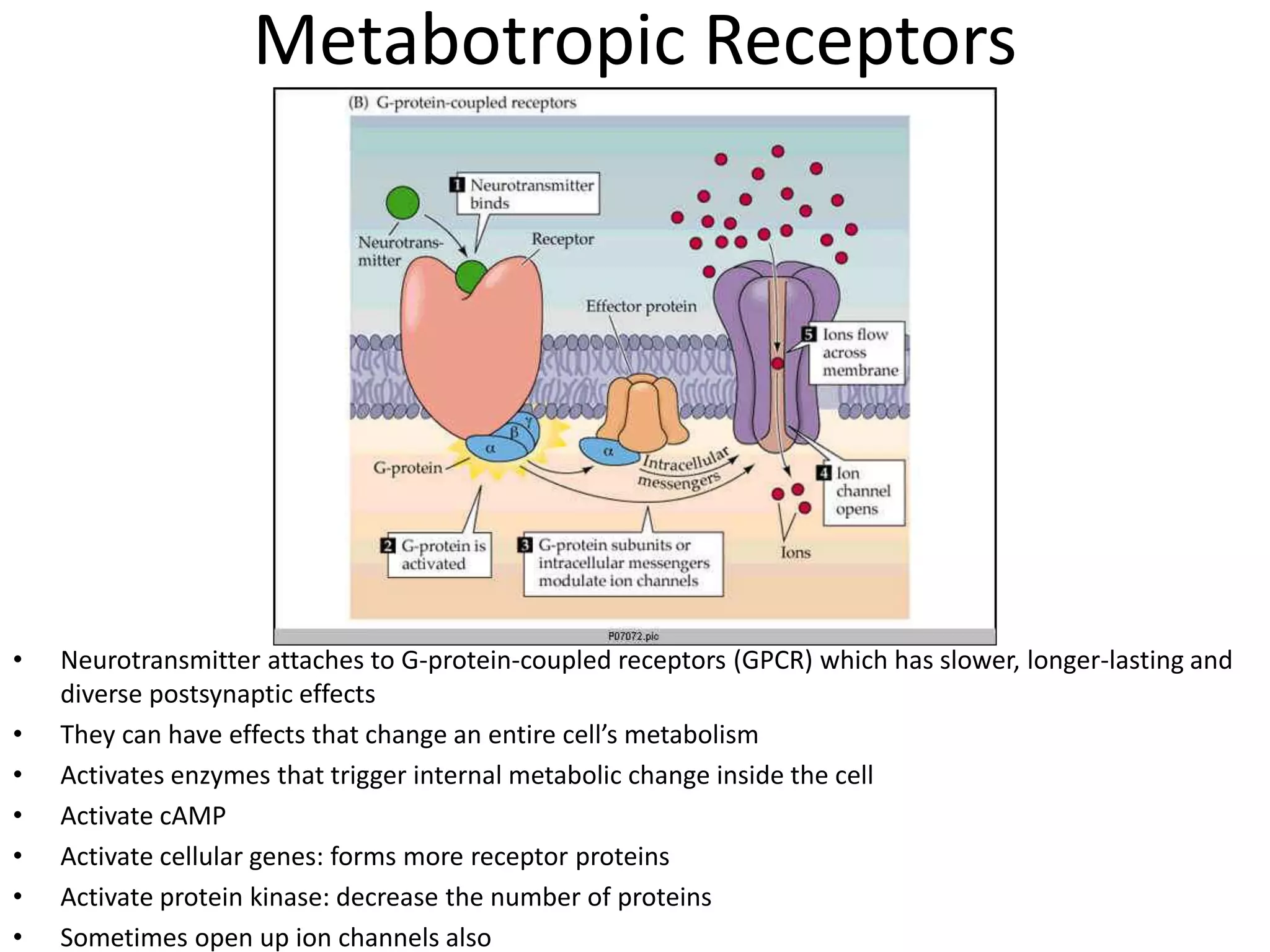
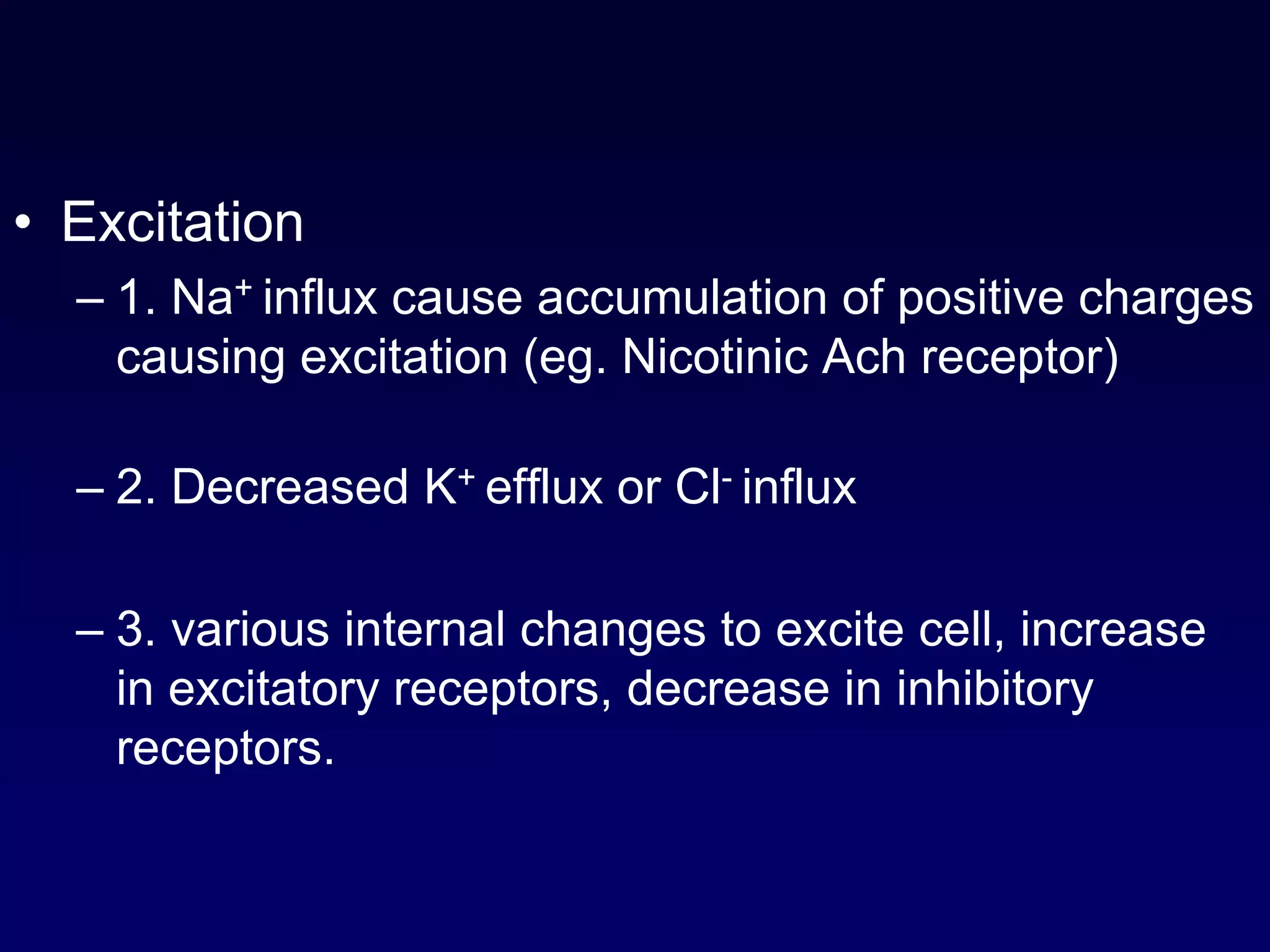
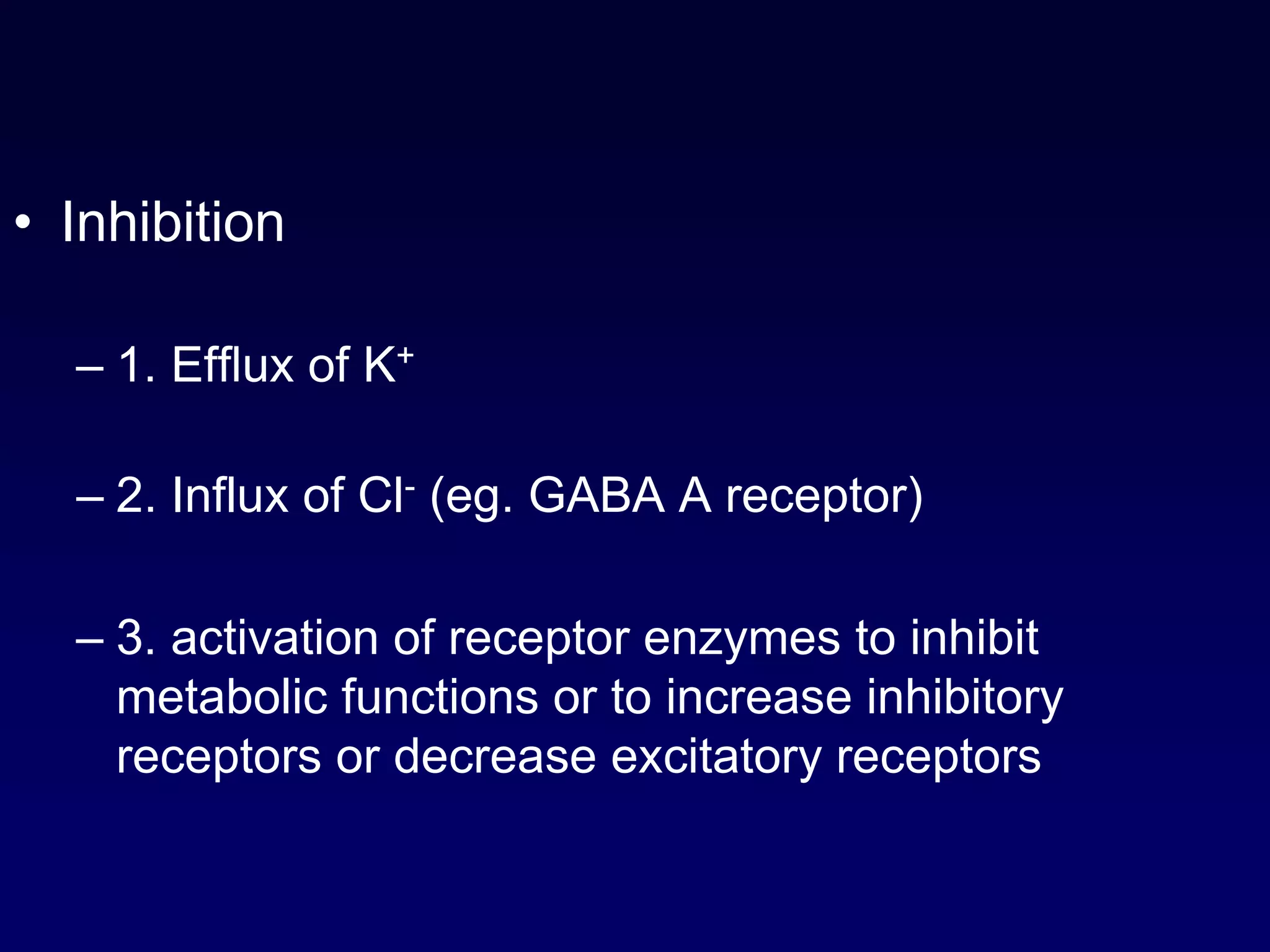
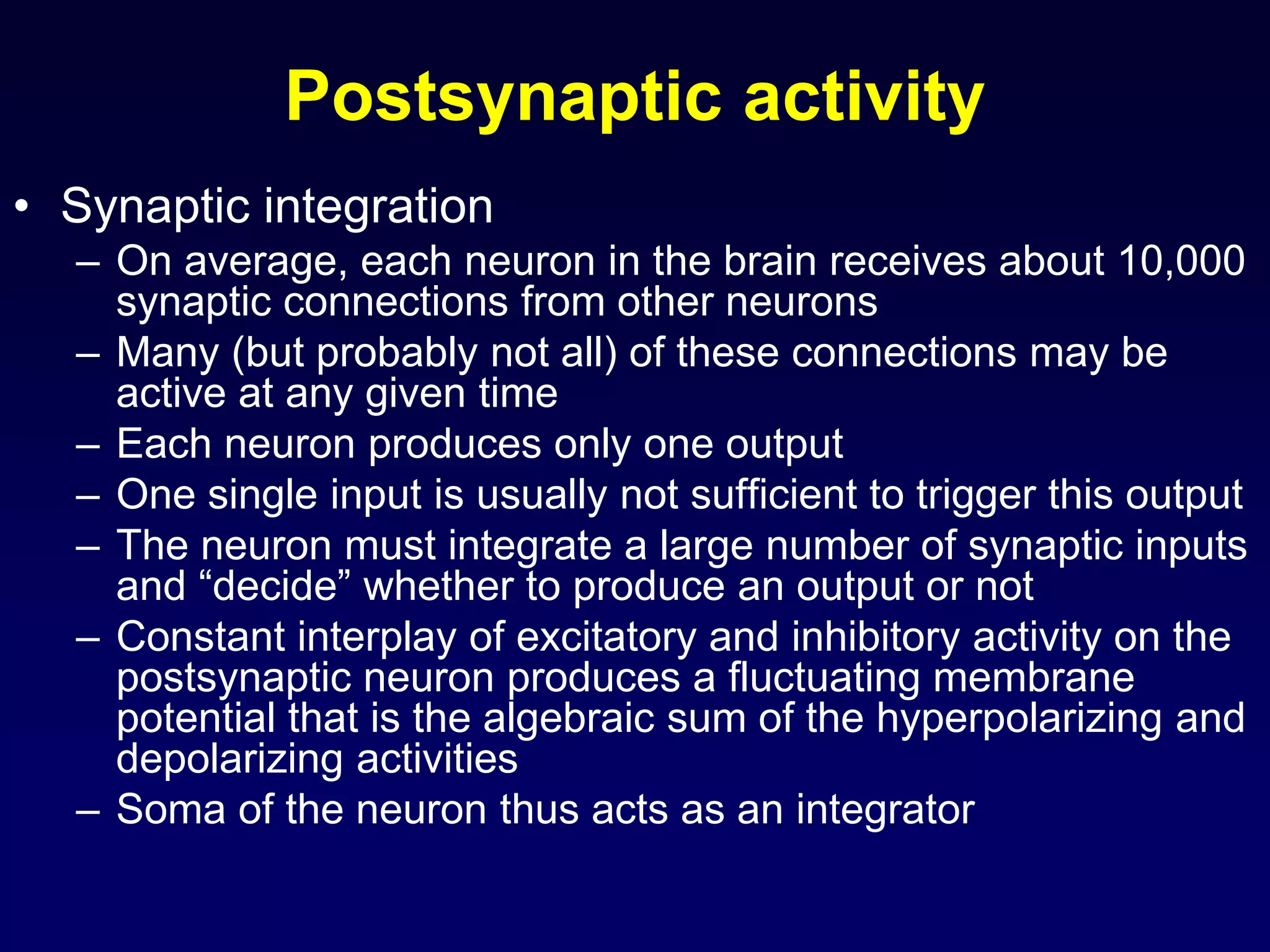
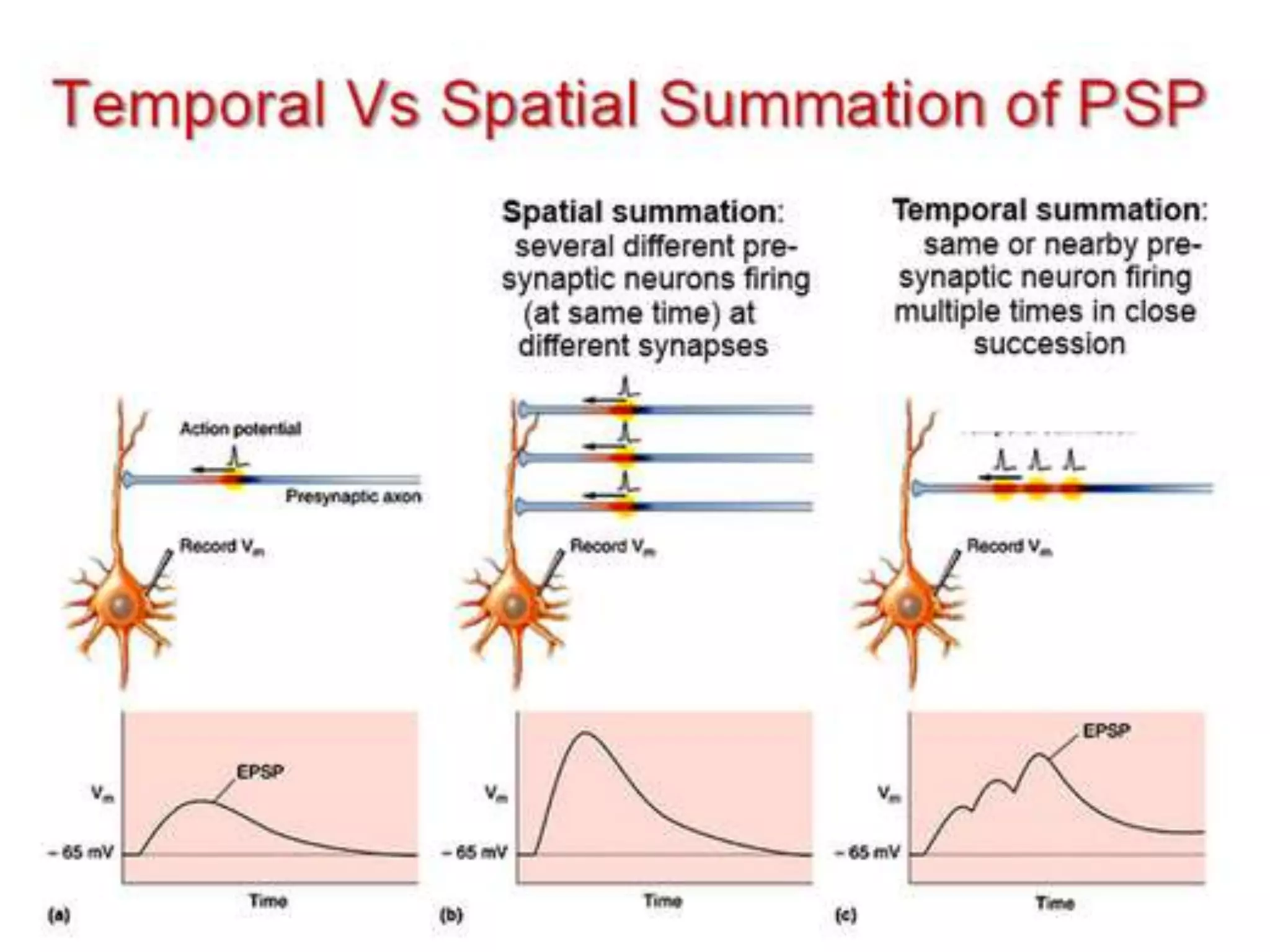
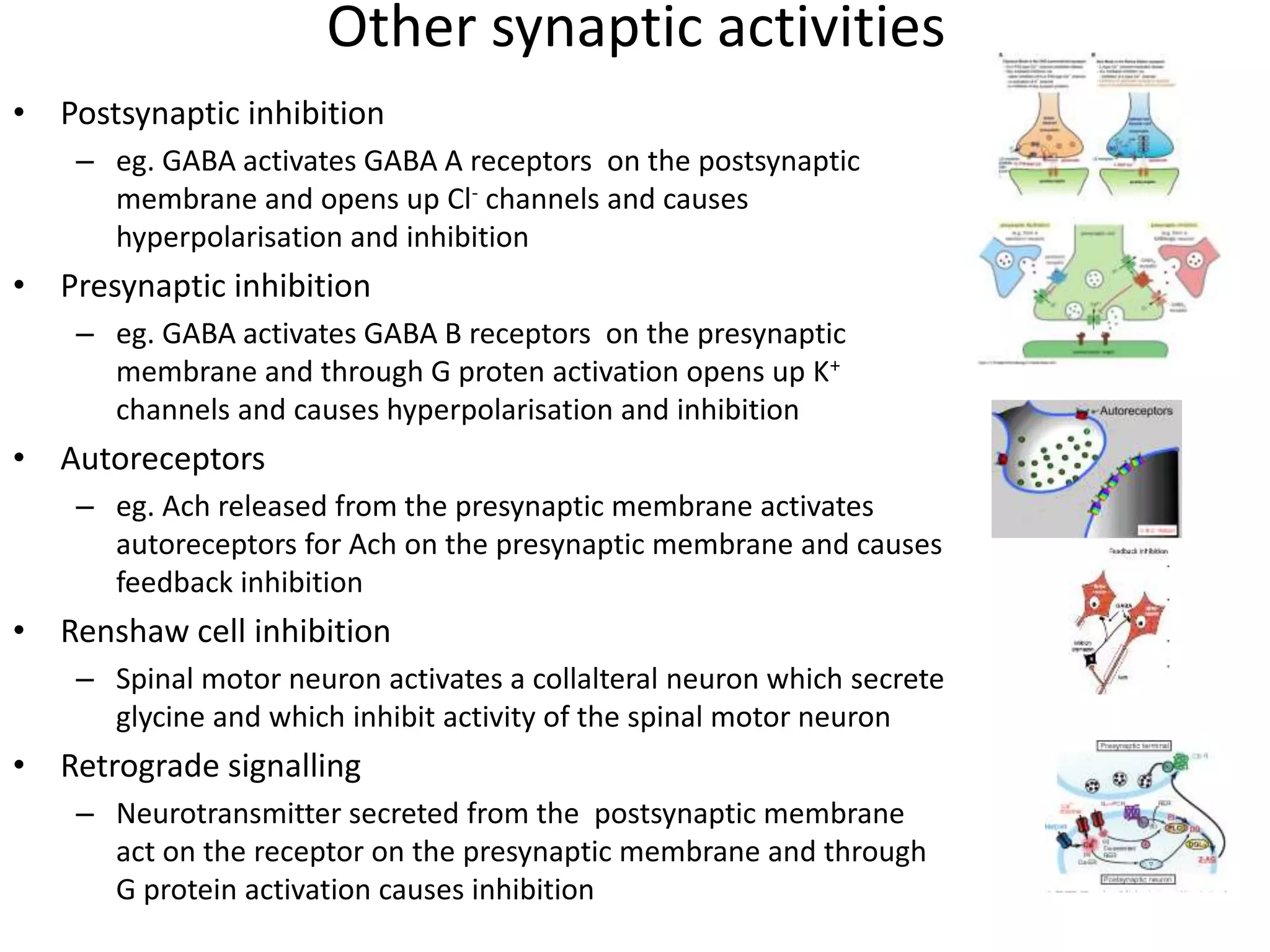
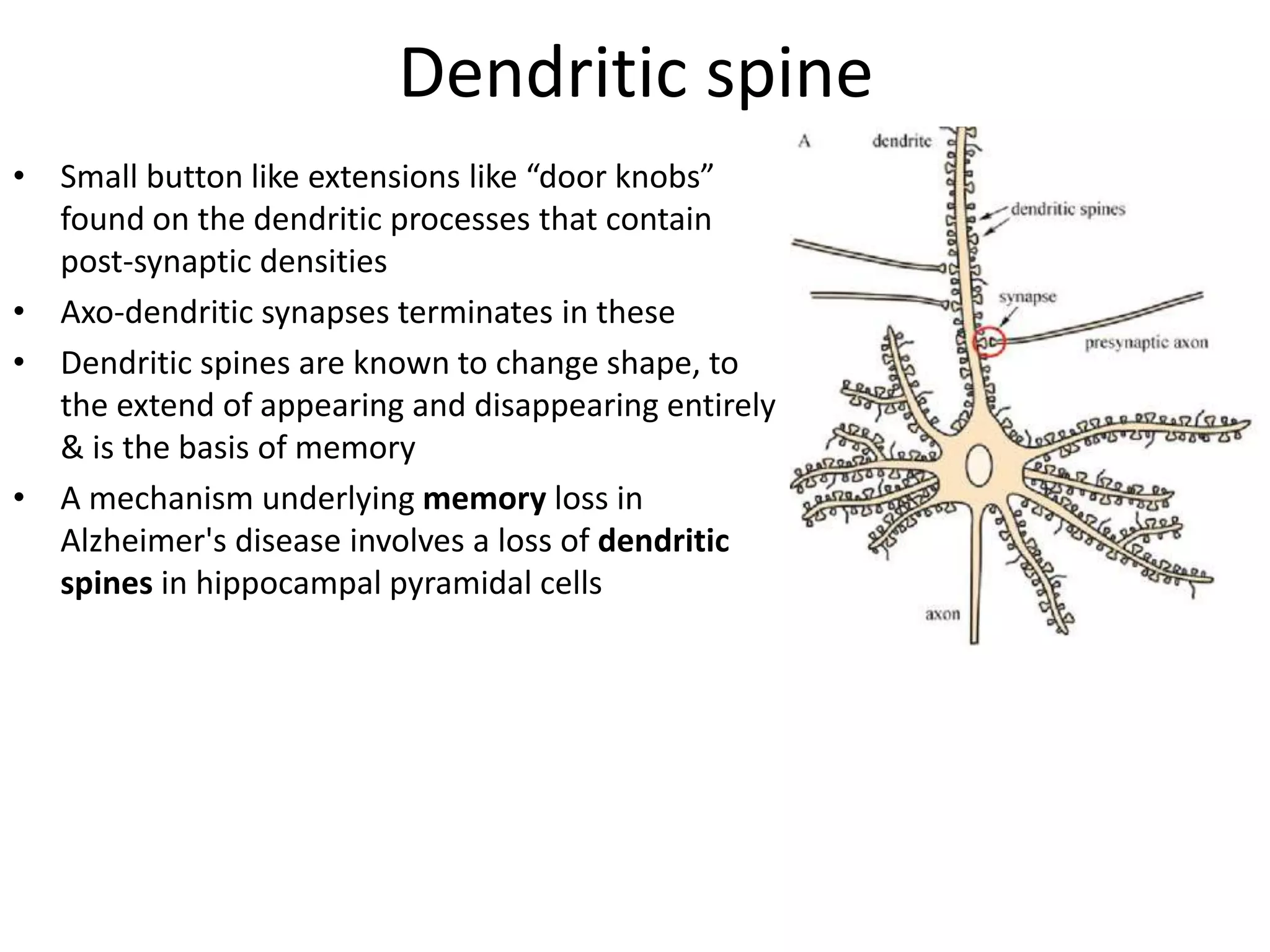
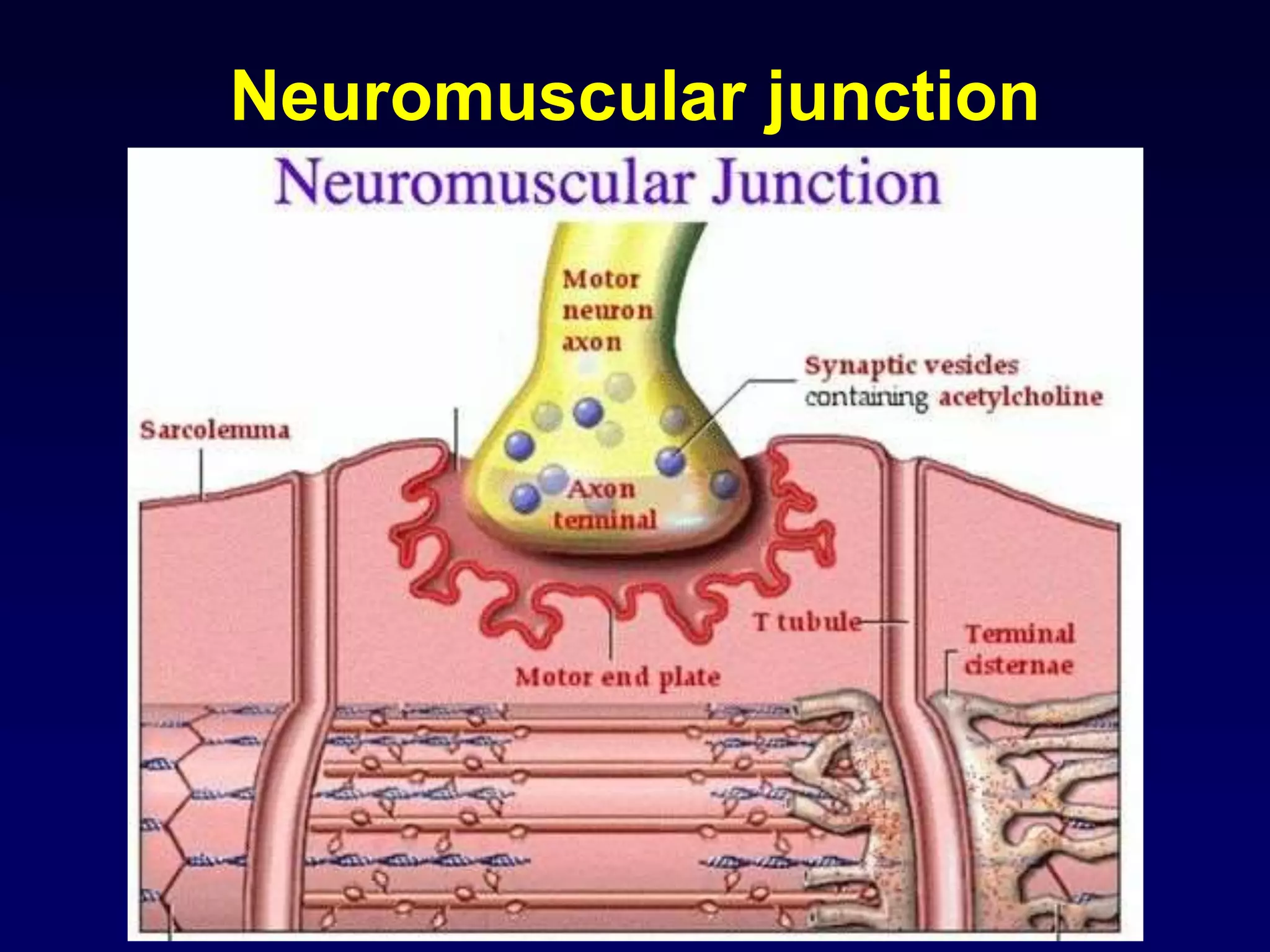
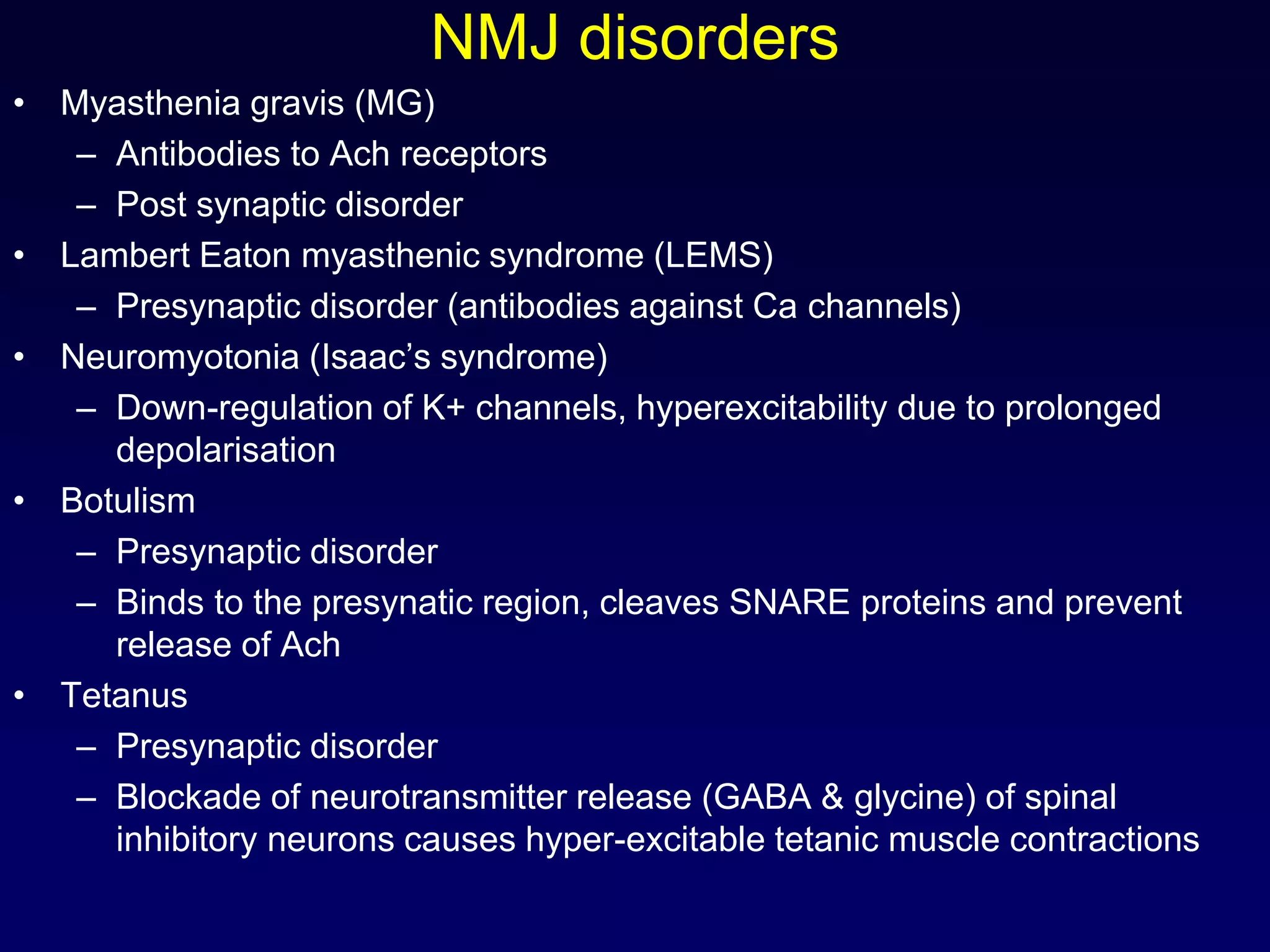
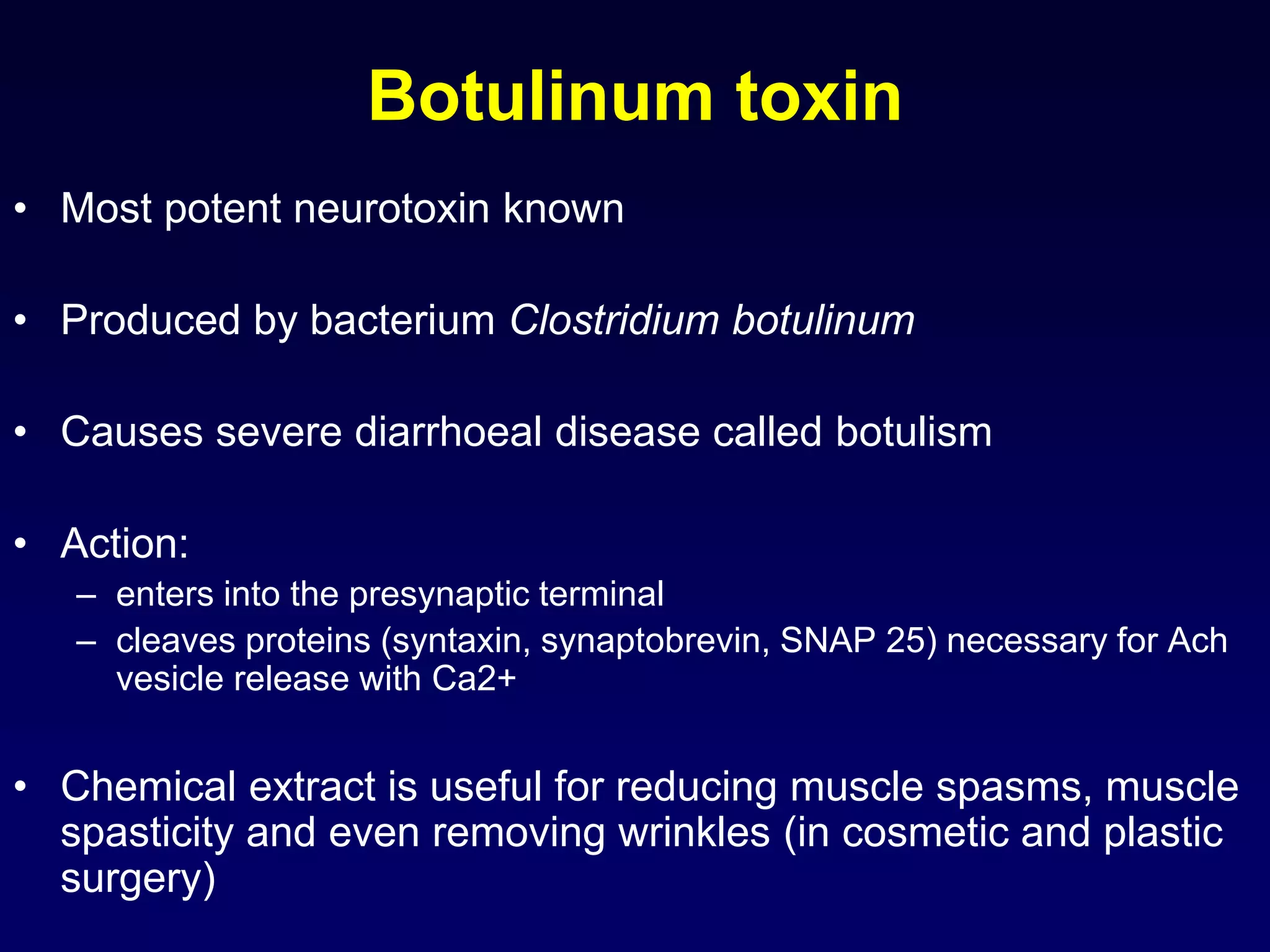
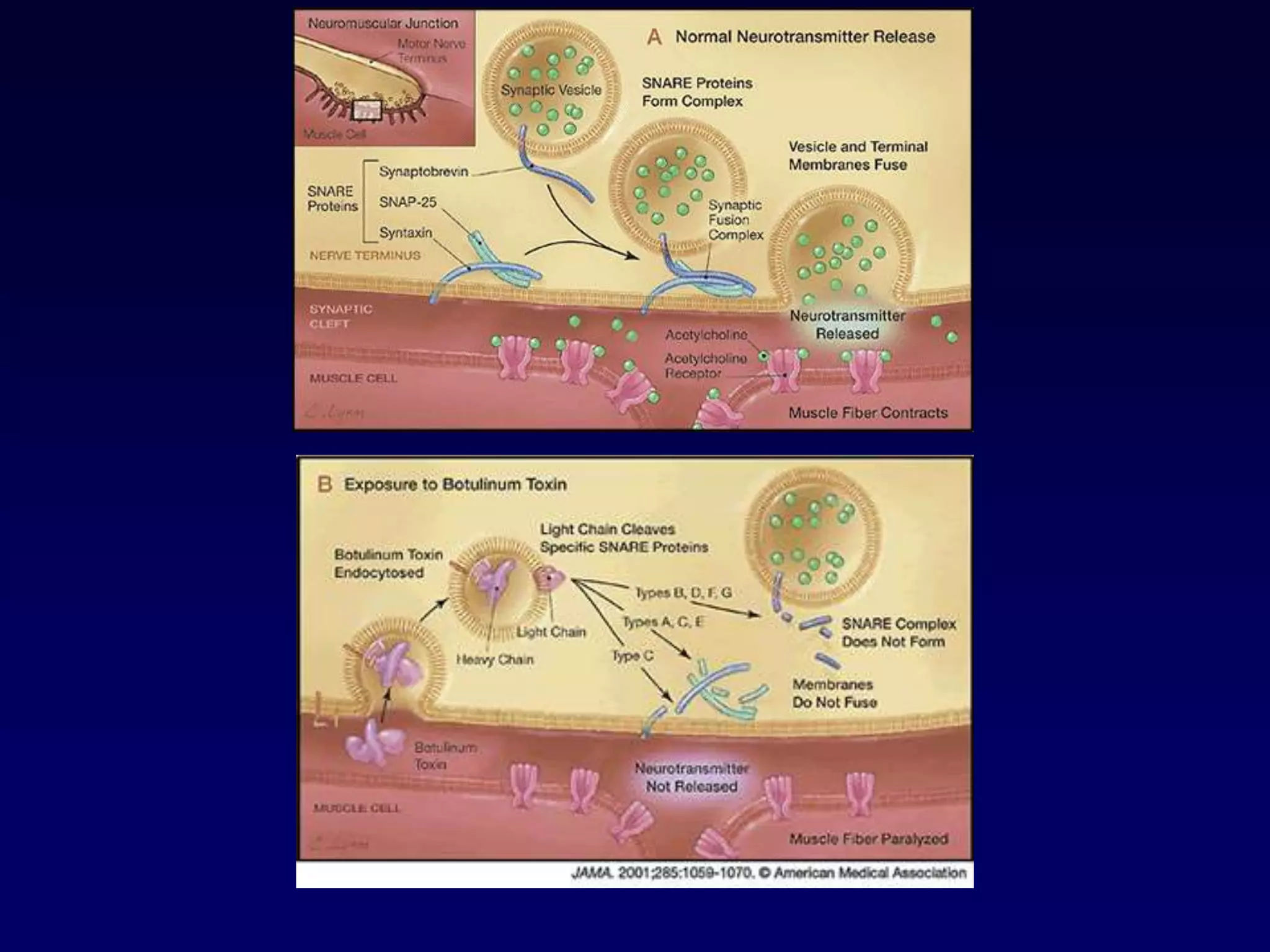
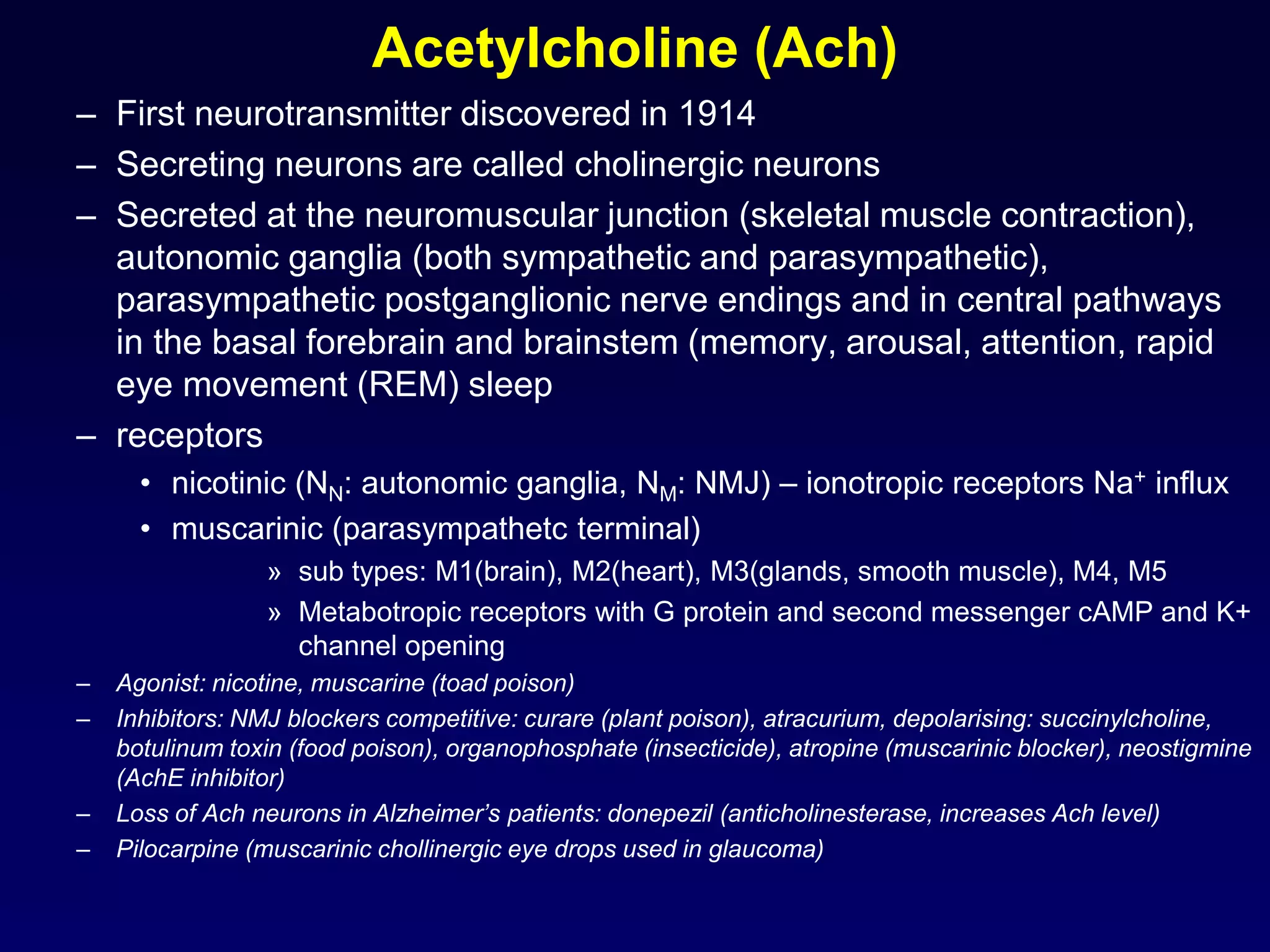
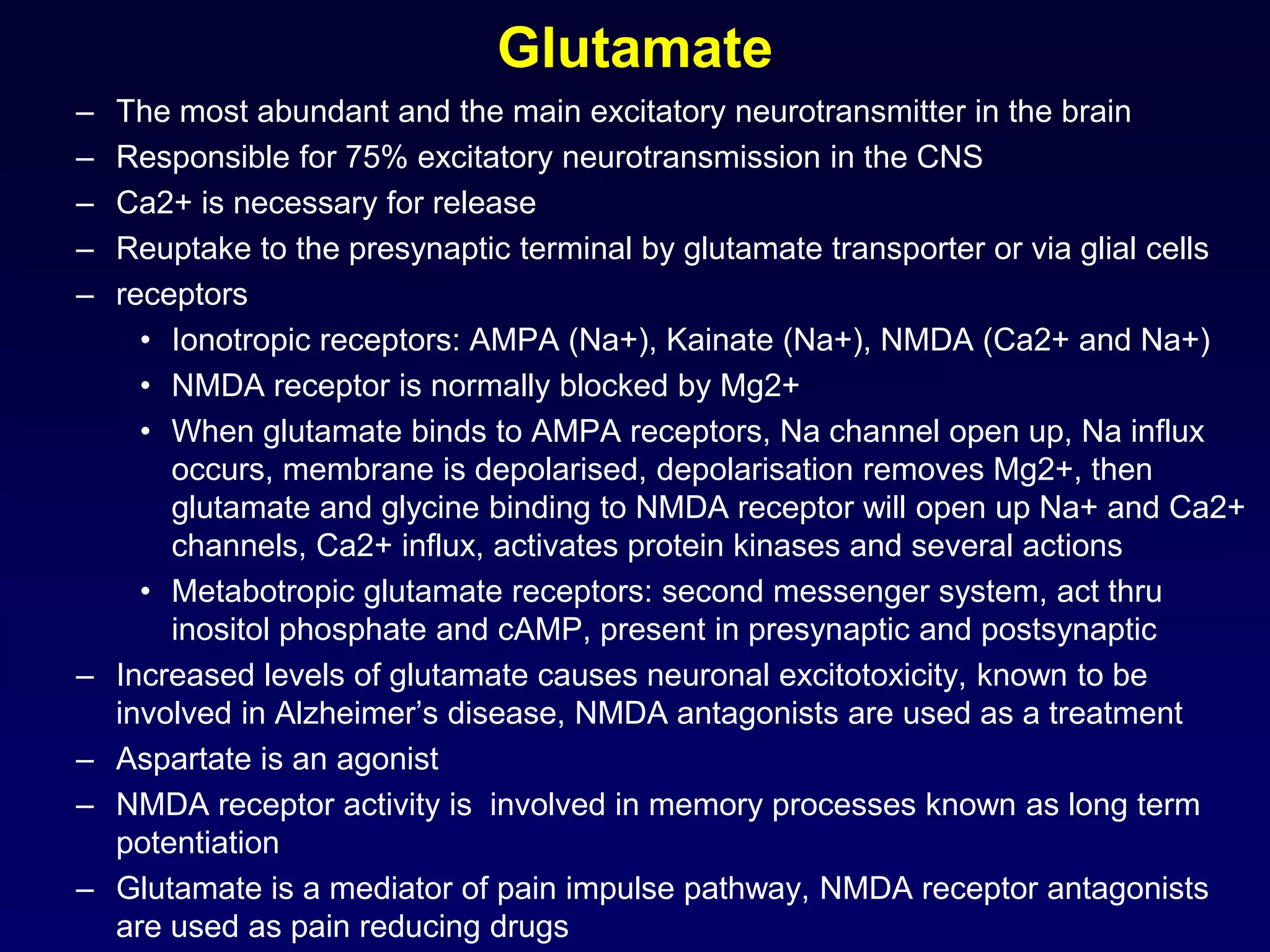
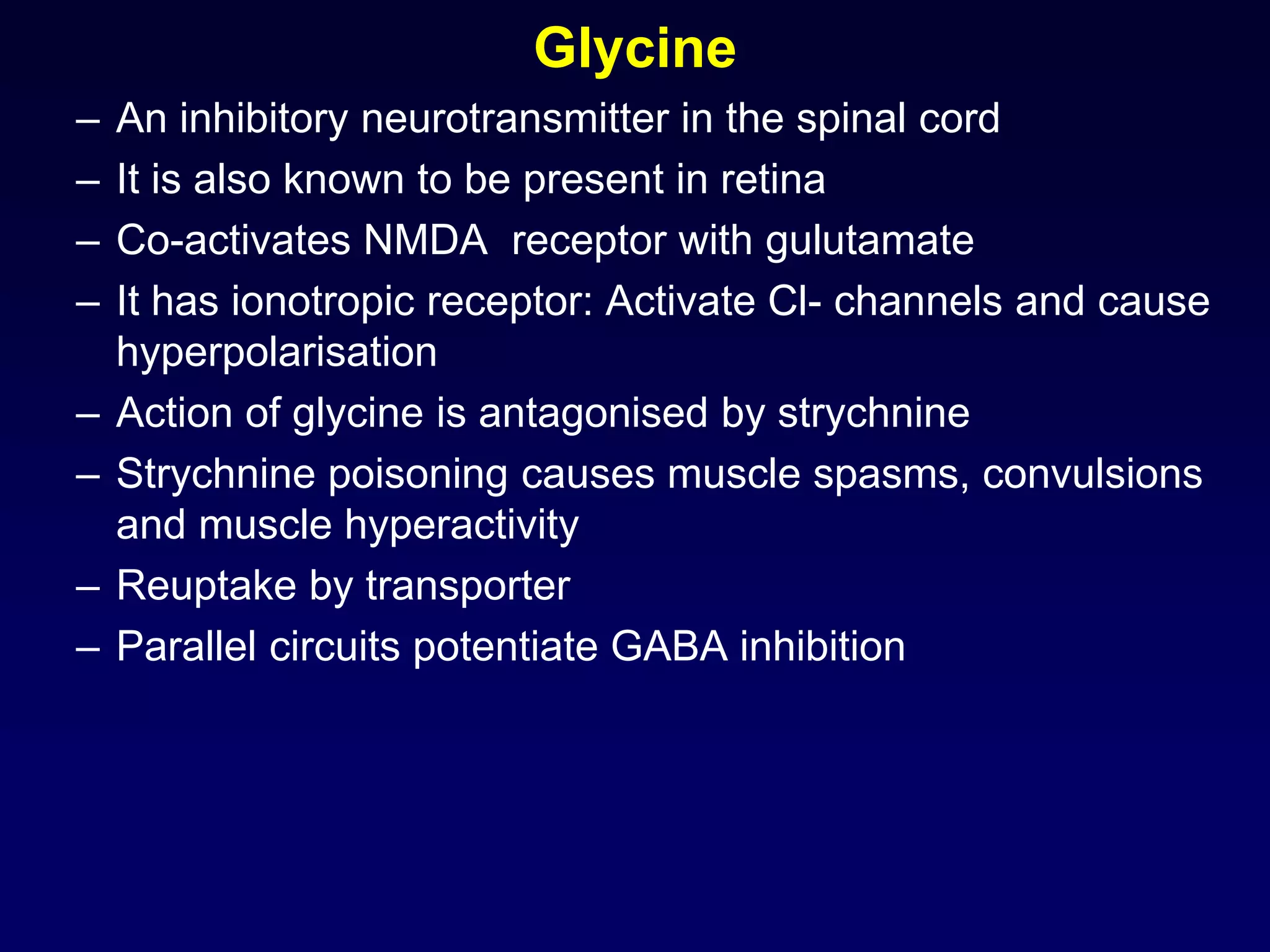
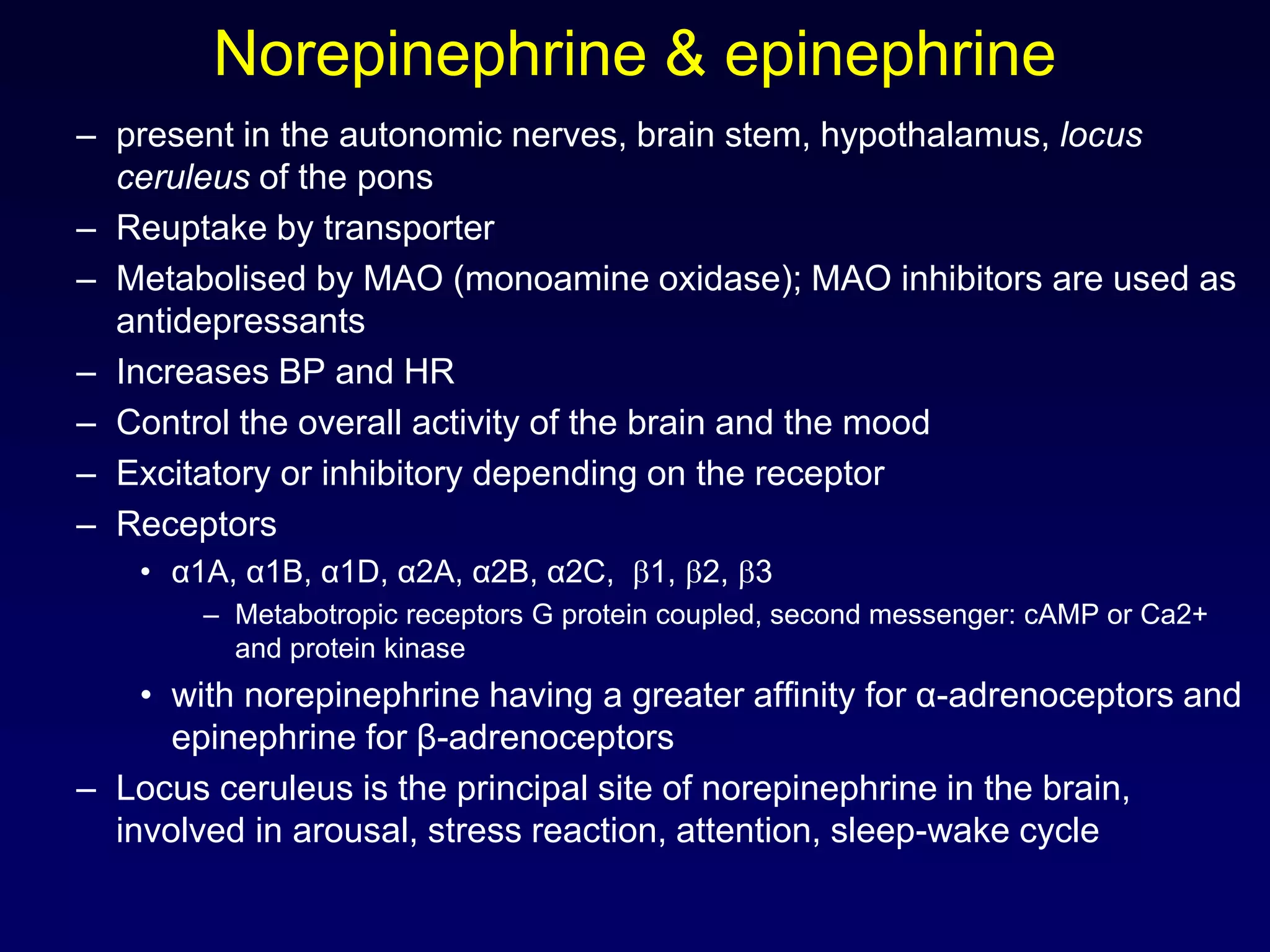
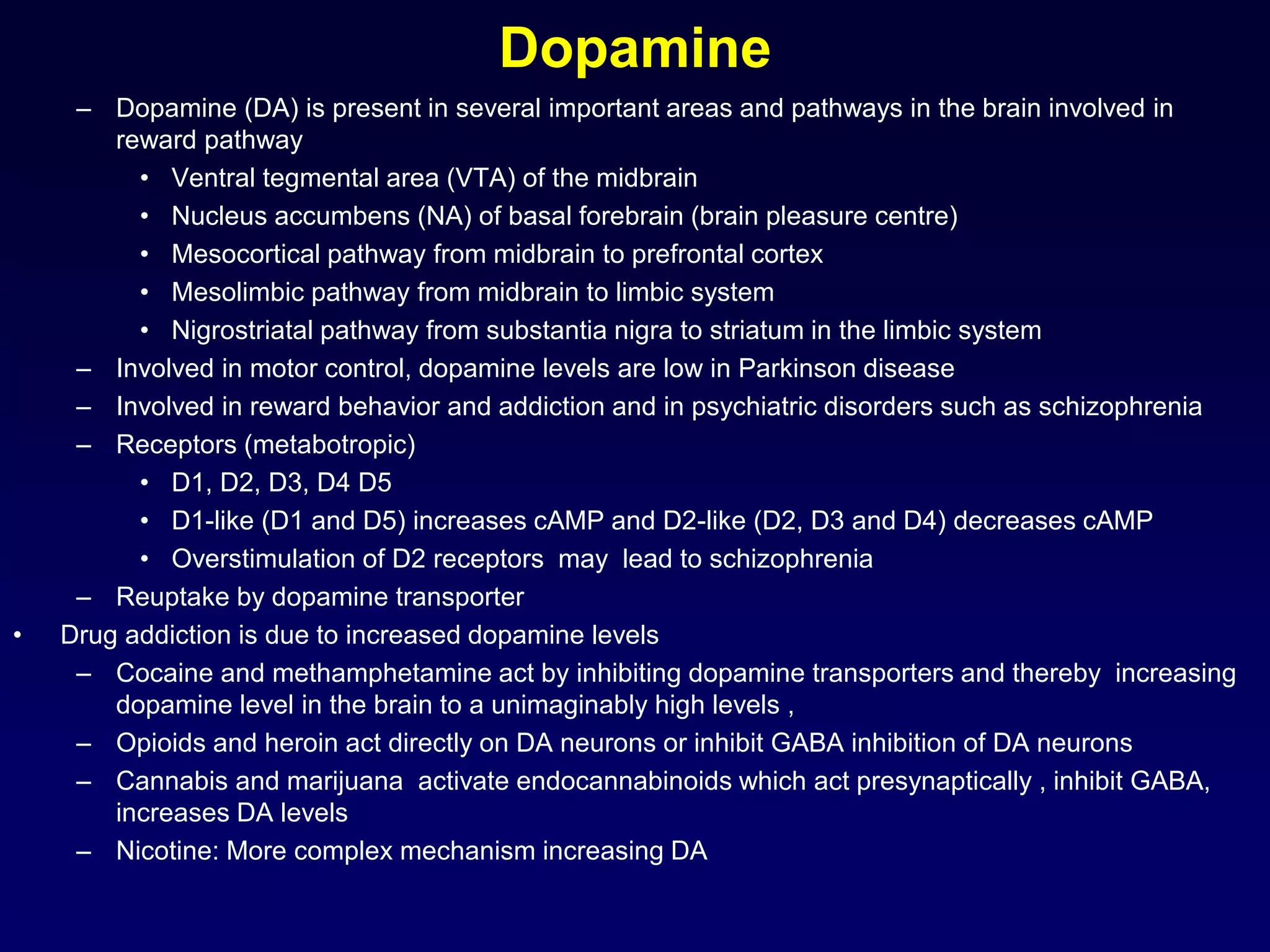
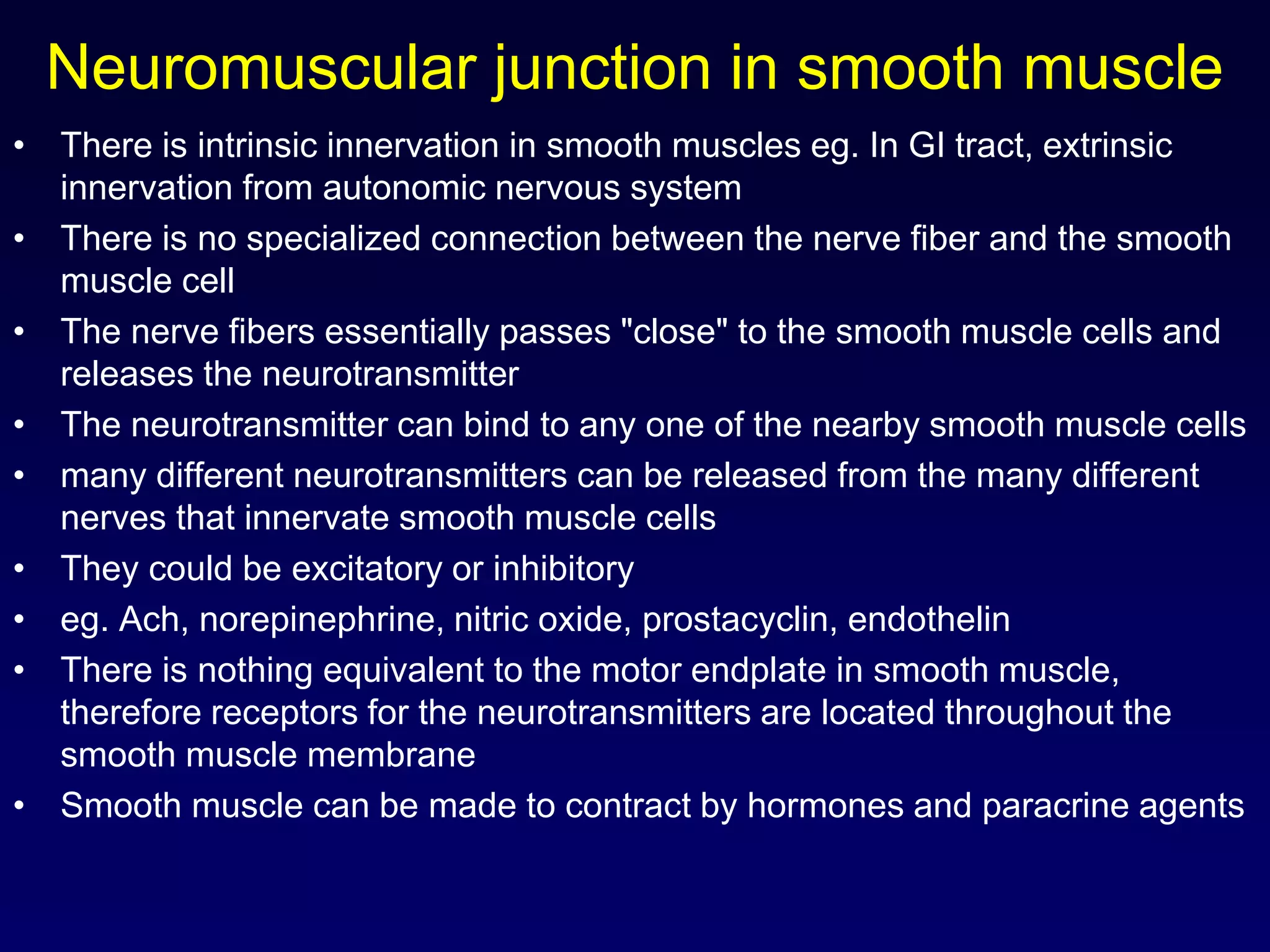
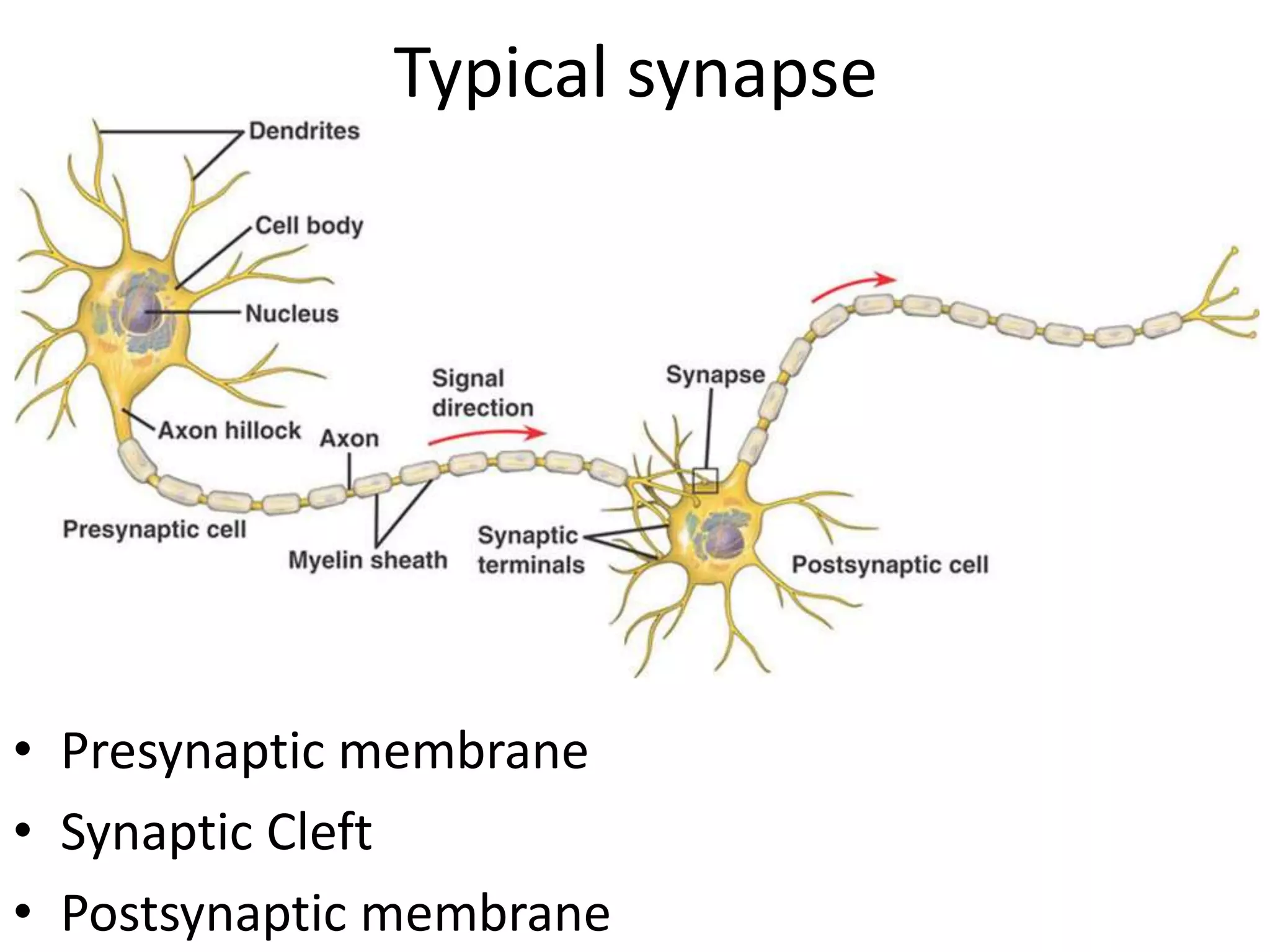
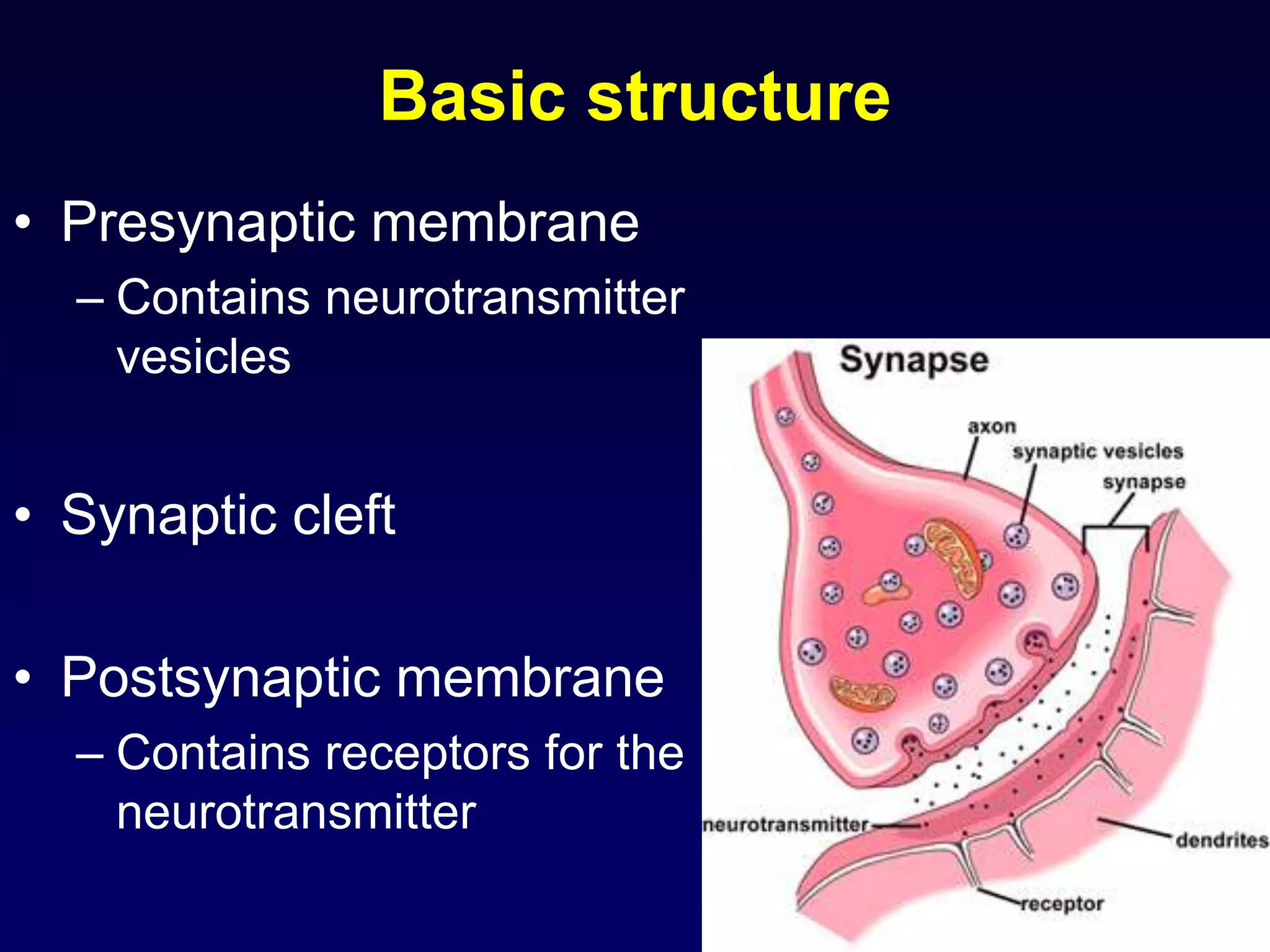
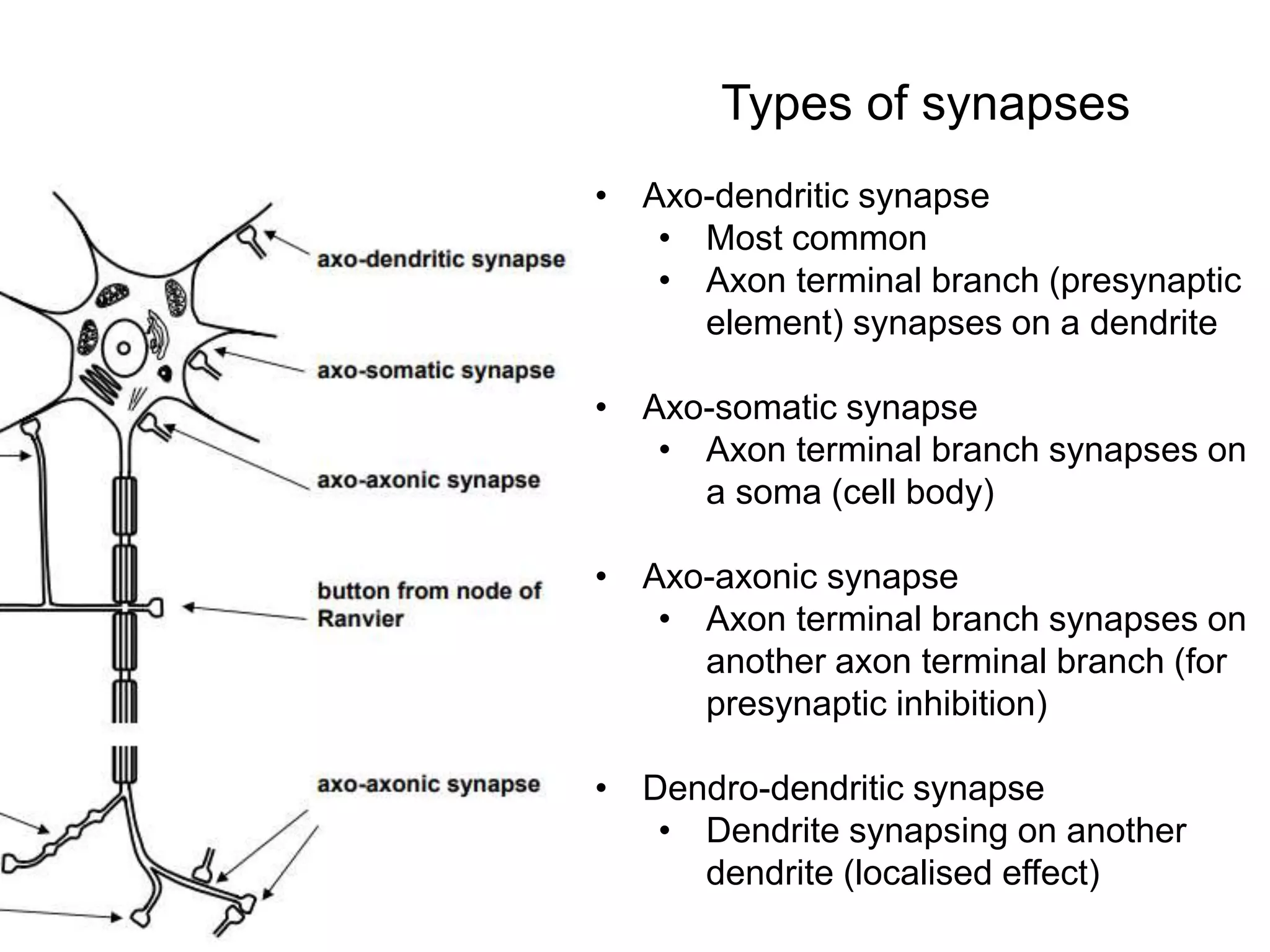
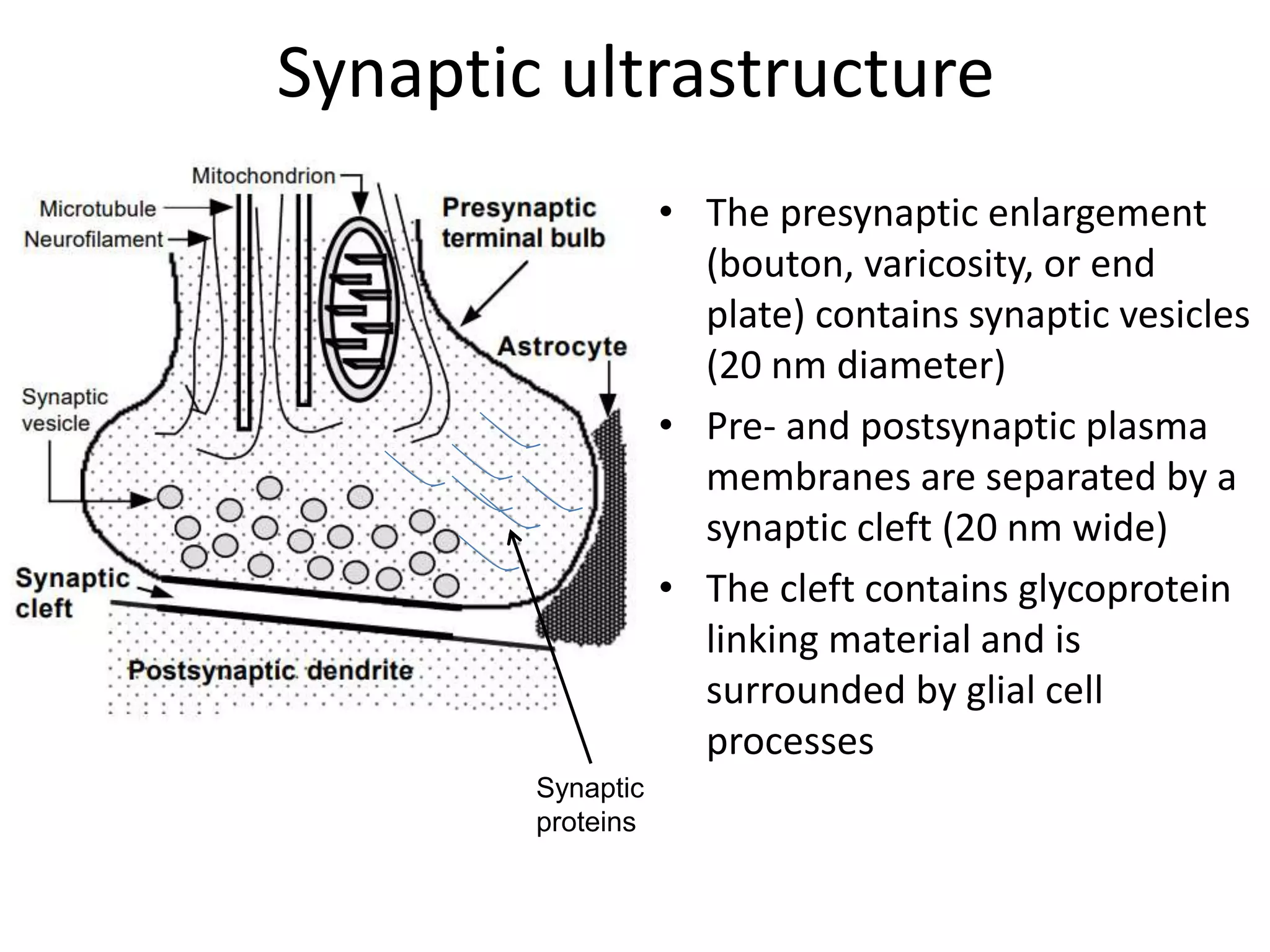
This document discusses synaptic transmission and neurotransmitters. It begins by describing the structure and function of synapses, including the roles of presynaptic and postsynaptic membranes. It then explains excitatory and inhibitory postsynaptic potentials. The document also discusses the neuromuscular junction, how acetylcholine is released and binds to nicotinic receptors to trigger muscle contraction. Finally, it outlines several major neurotransmitters - acetylcholine, glutamate, and GABA - including their receptors, mechanisms of action, and effects on synaptic transmission.

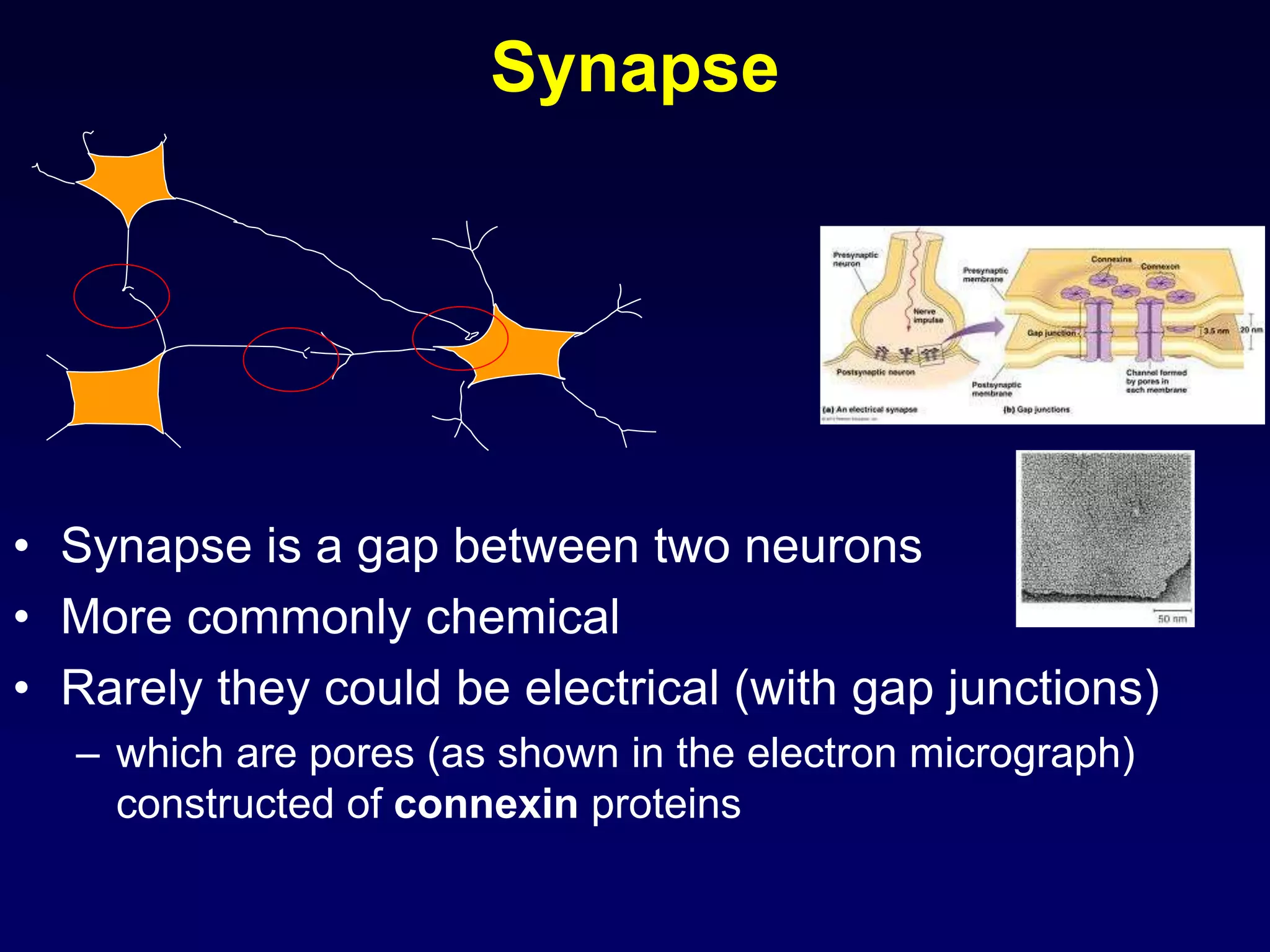
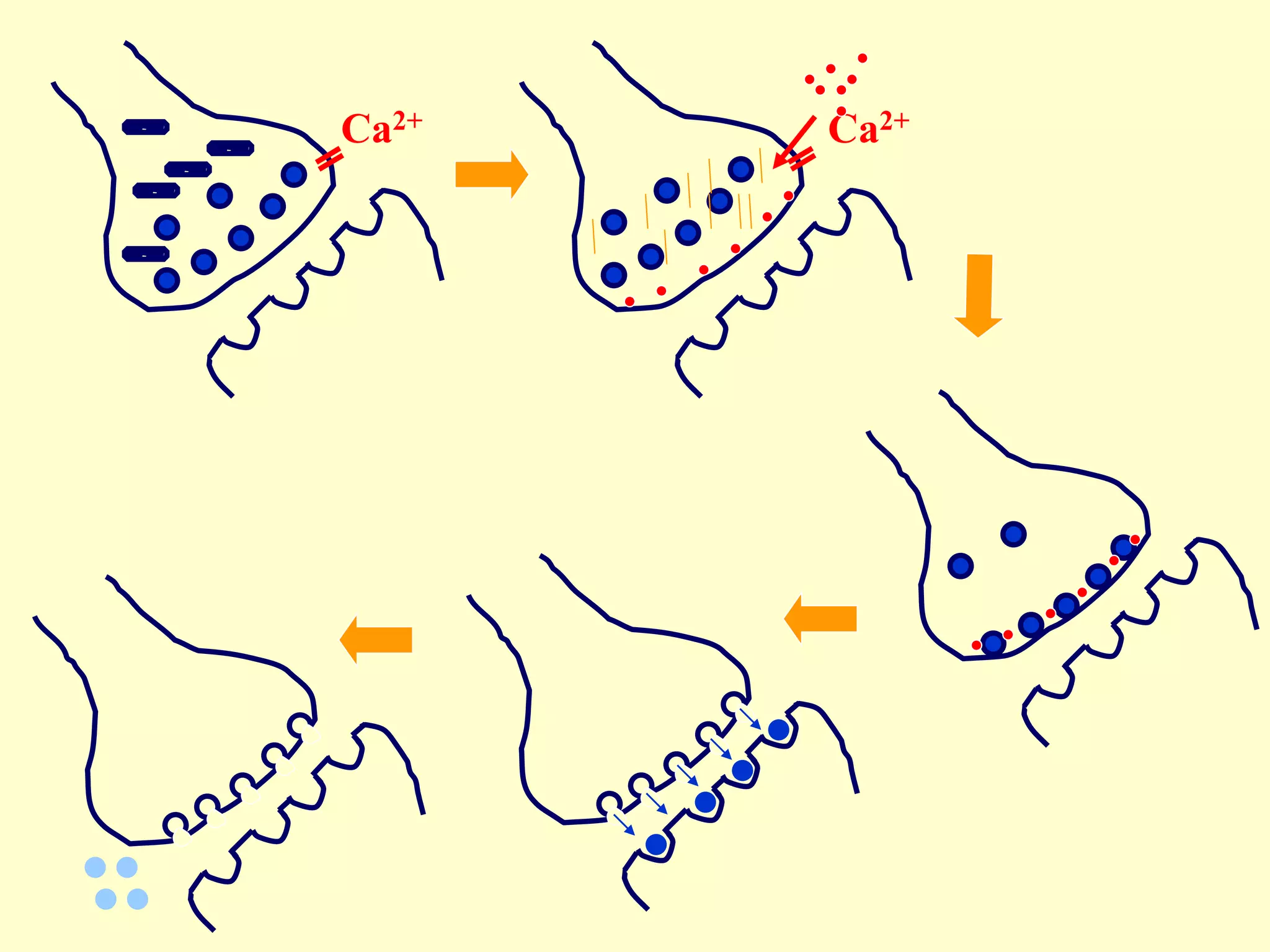
![Details of presynaptic events
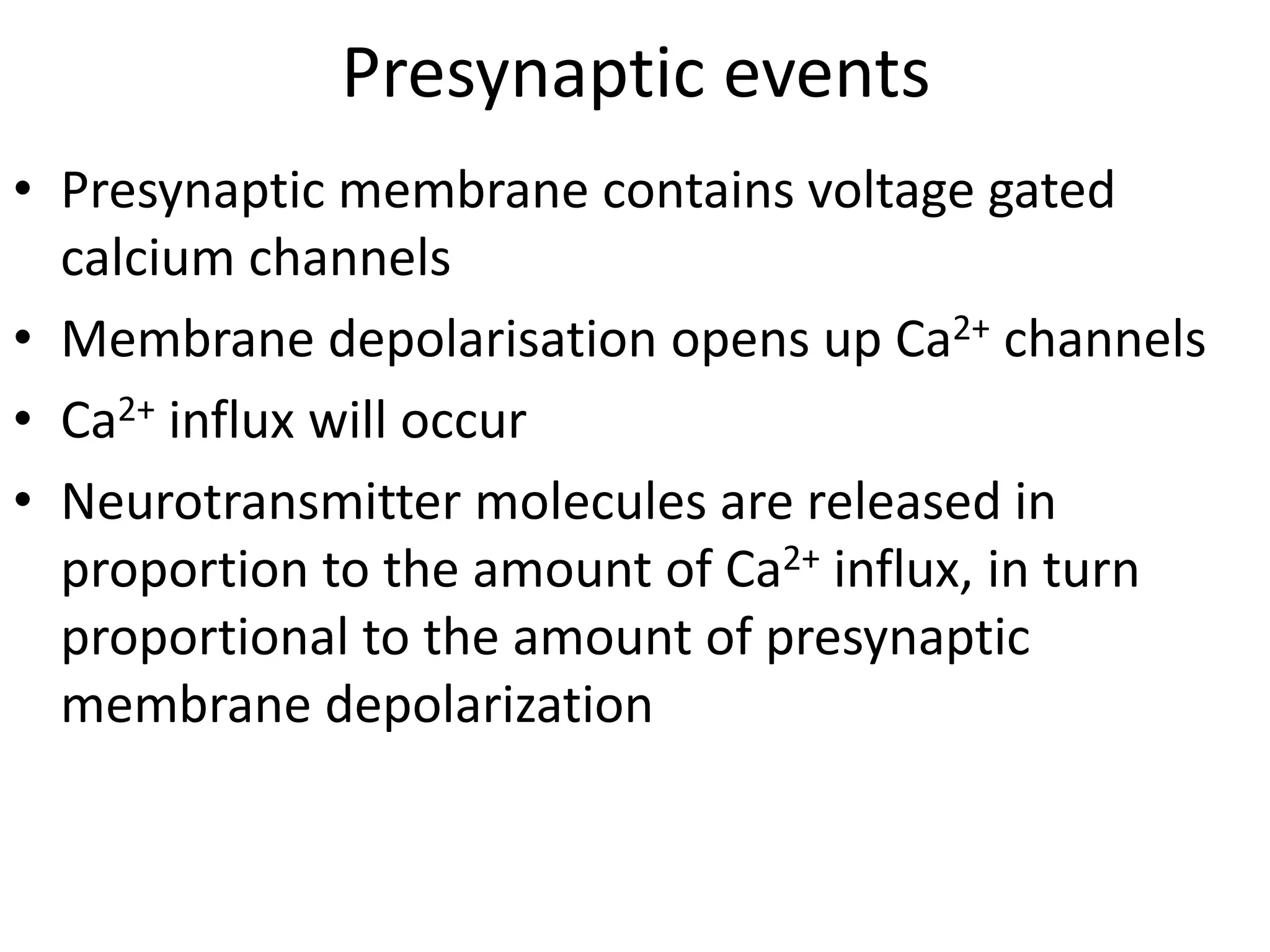
• in the resting state, the presynaptic membrane has resting membrane potential
• when an action potential arrives at the end of the axon
• the adjacent presynaptic membrane is depolarised
• voltage-gated Ca2+ channels open and allow Ca2+ influx (driven by [Ca2+] gradient)
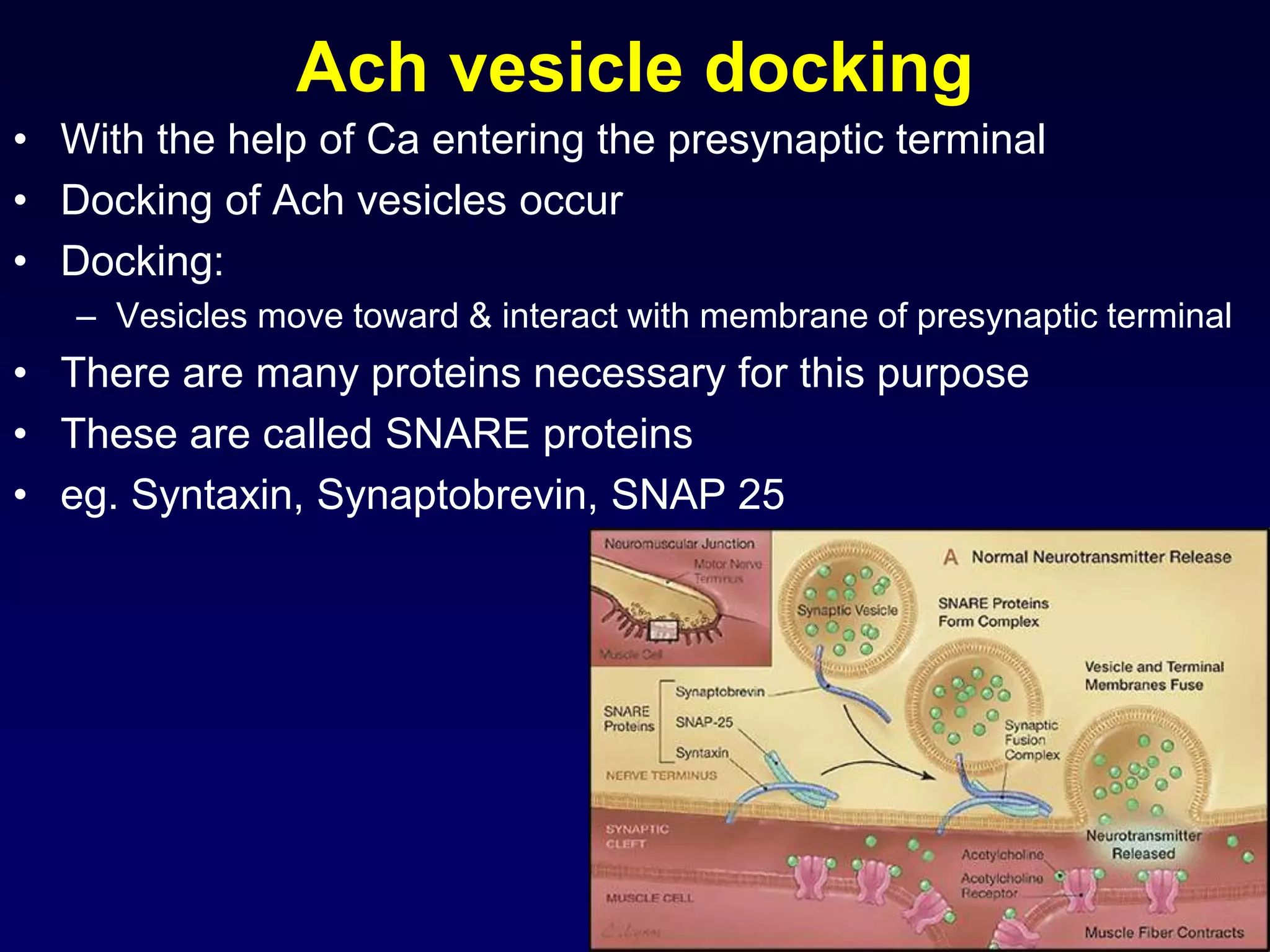
• elevated [Ca2+] activates synaptic proteins
(SNARE proteins: Synaptobrevin, Syntaxin, SNAP 25) and triggers vesicle
mobilization and docking with the plasma membrane
• vesicles fuse with presynaptic plasma membrane and release neurotransmitter
molecules (about 5,000 per vesicle) by exocytosis
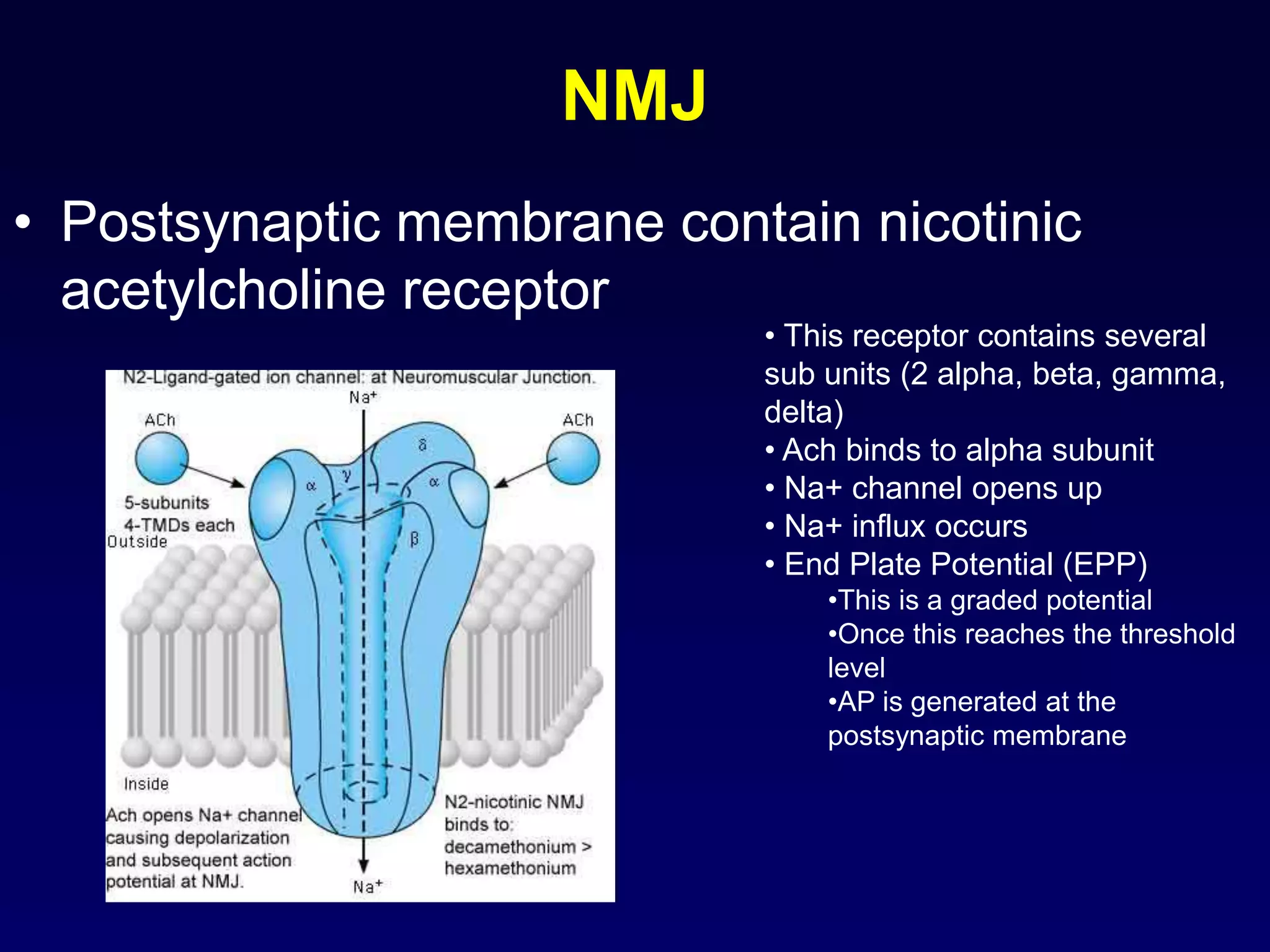
• neurotransmitter molecules diffuse across the cleft & bind with postsynaptic
receptor proteins
• neurotransmitter molecules are eliminated from synaptic clefts via pinocytotic
uptake by presynaptic or glial processes and/or via enzymatic degradation at the
postsynaptic membrane
• molecules are recycled
• subsequently, presynaptic plasma membrane repolarises](https://image.slidesharecdn.com/synapsenmjy1s12020slides-200113040550/75/Synapse-nmj-y1-s1-2020-slides-10-2048.jpg)