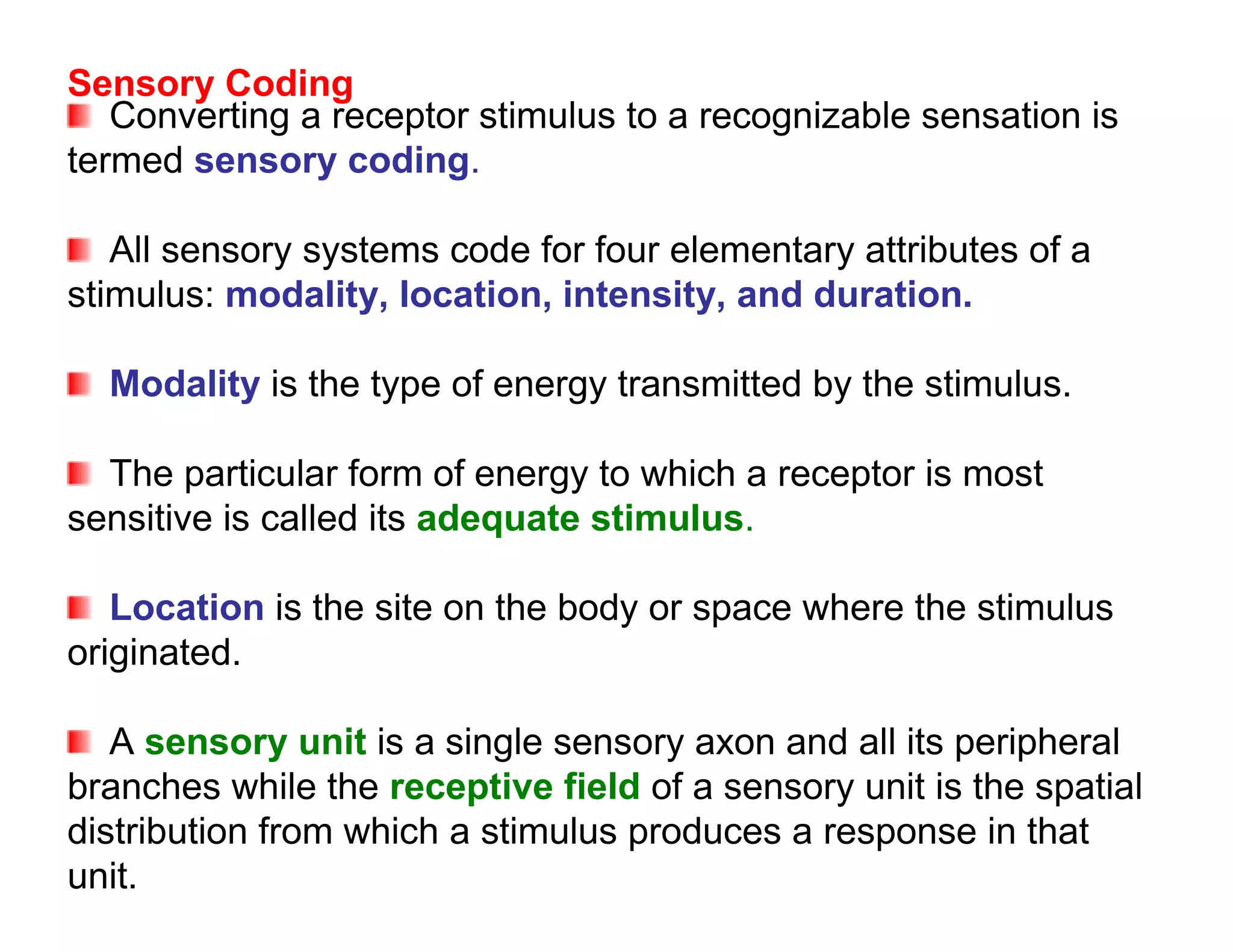
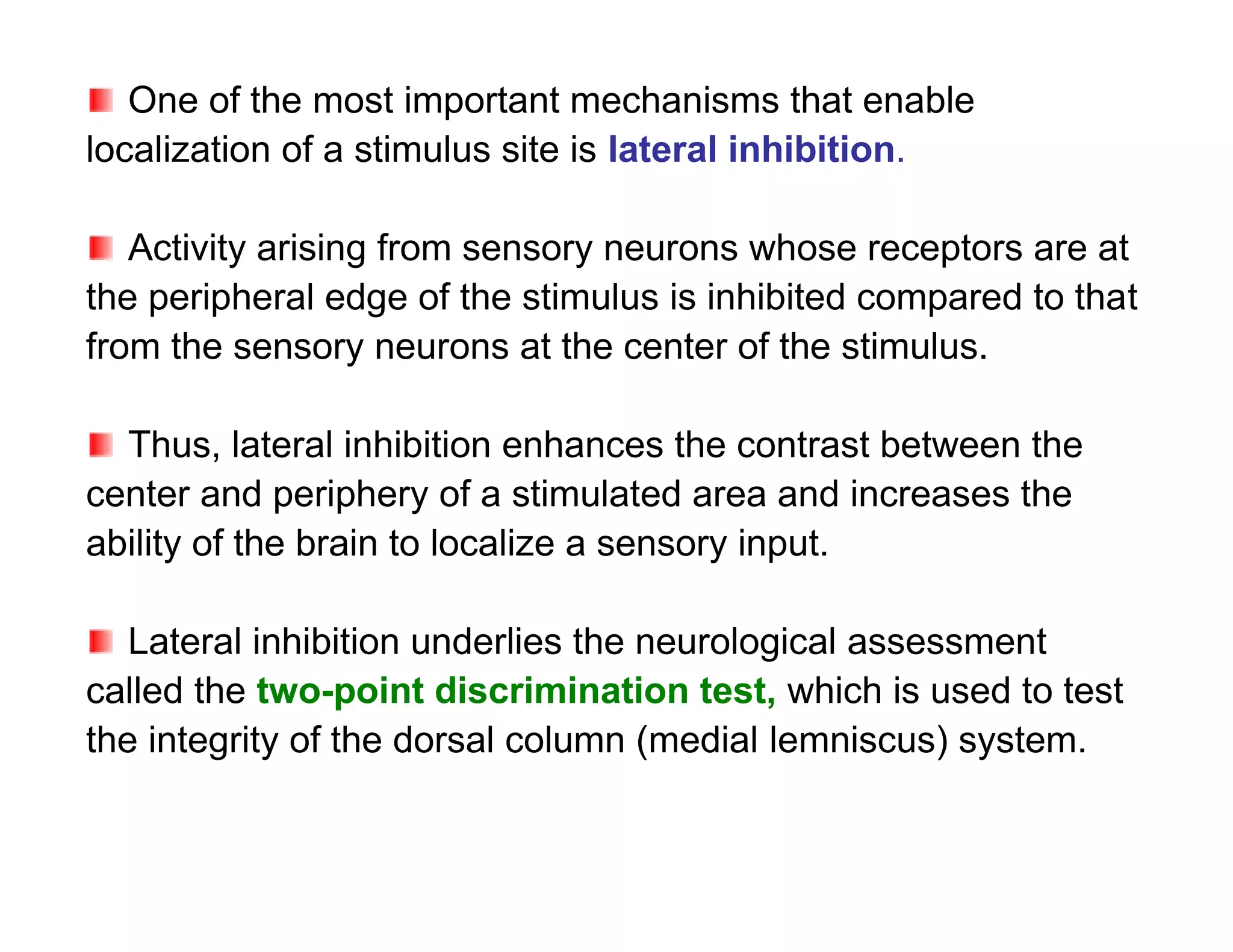
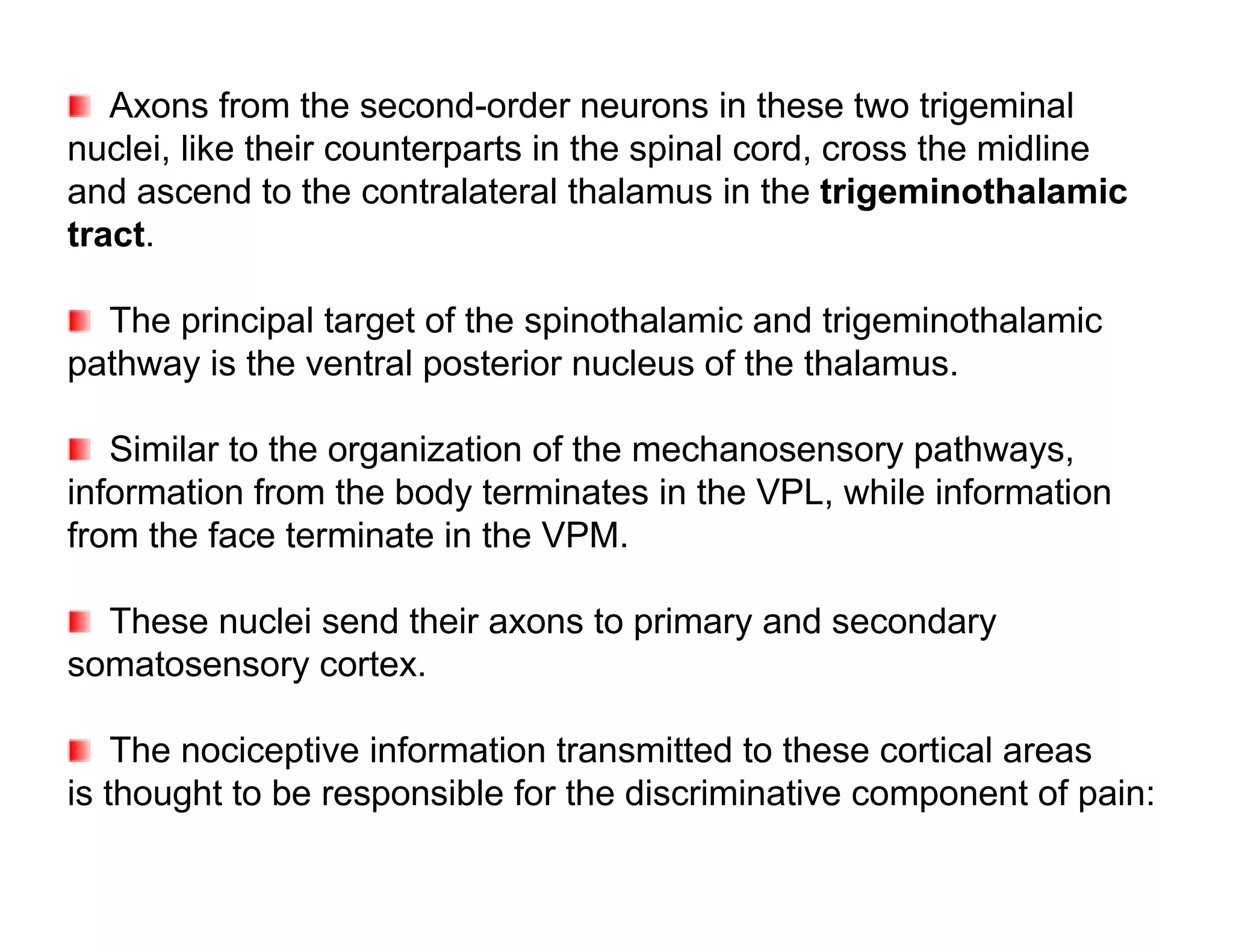
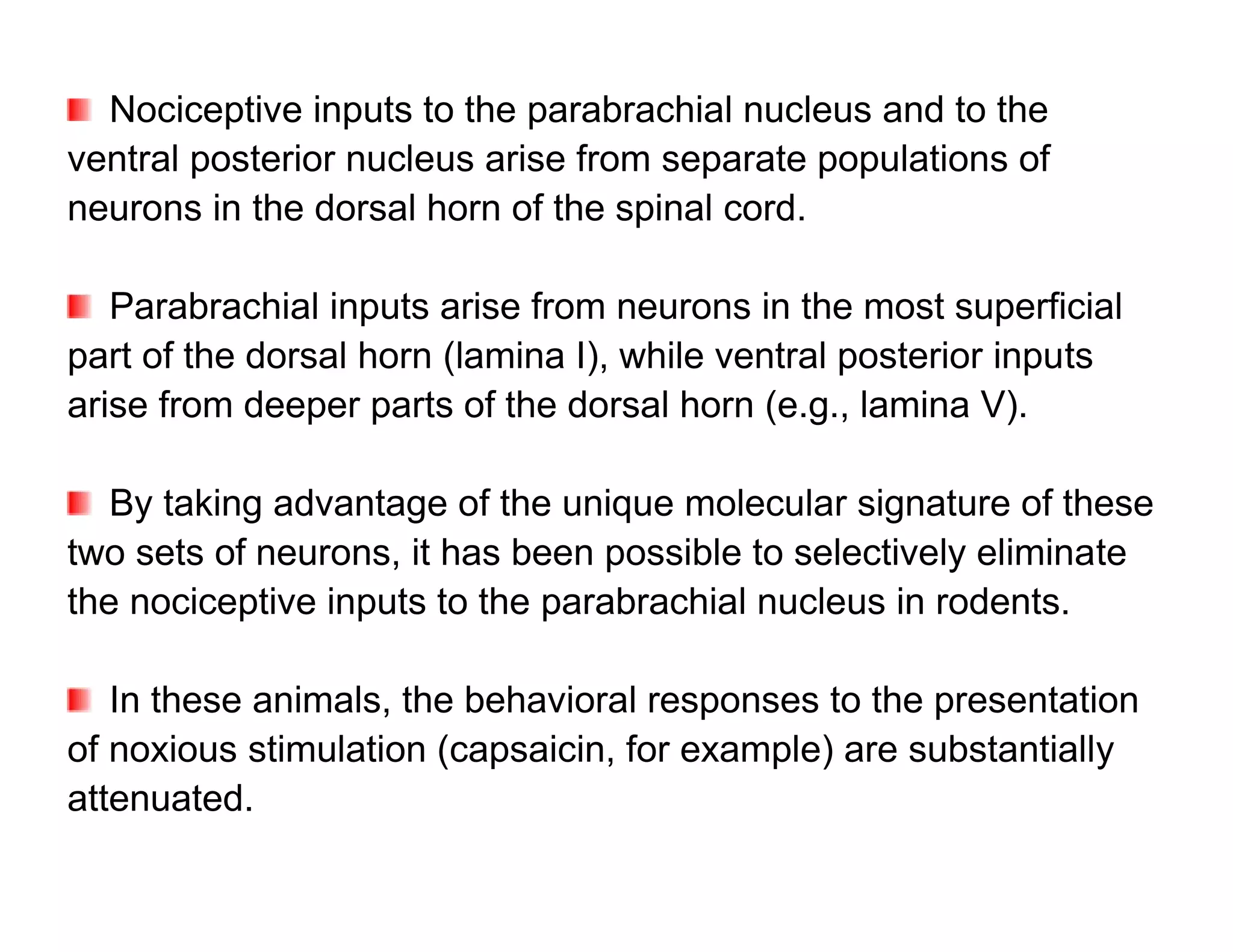
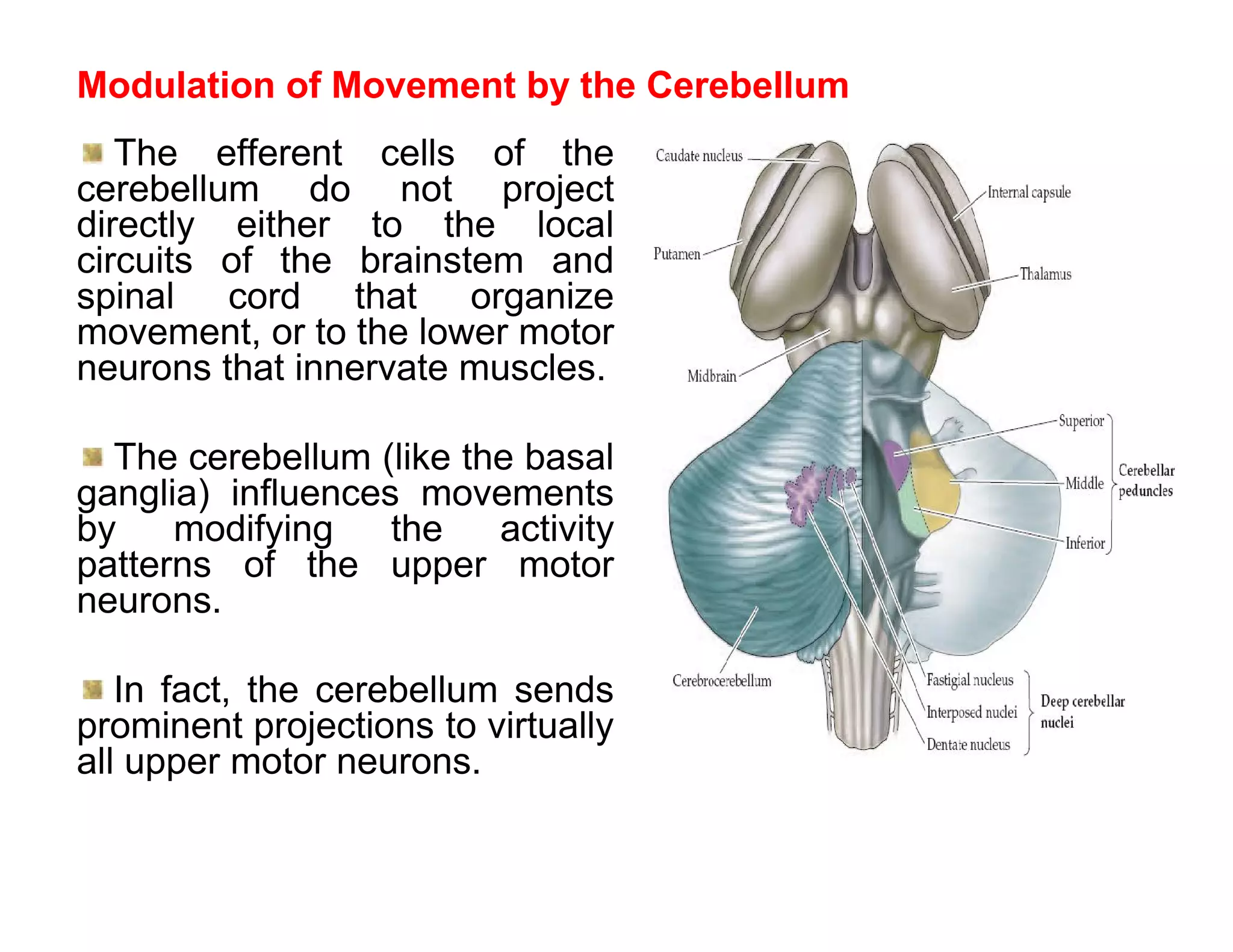
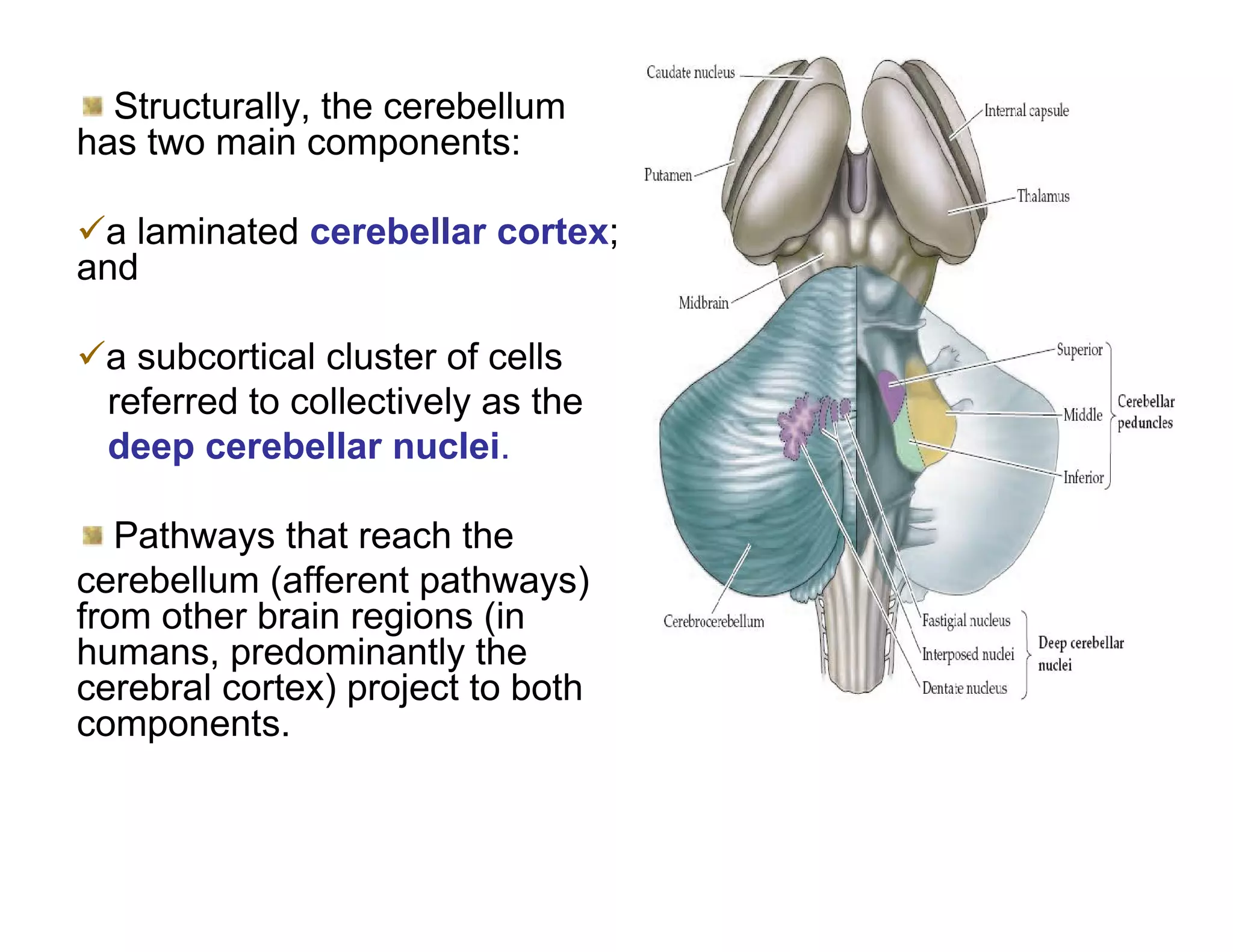
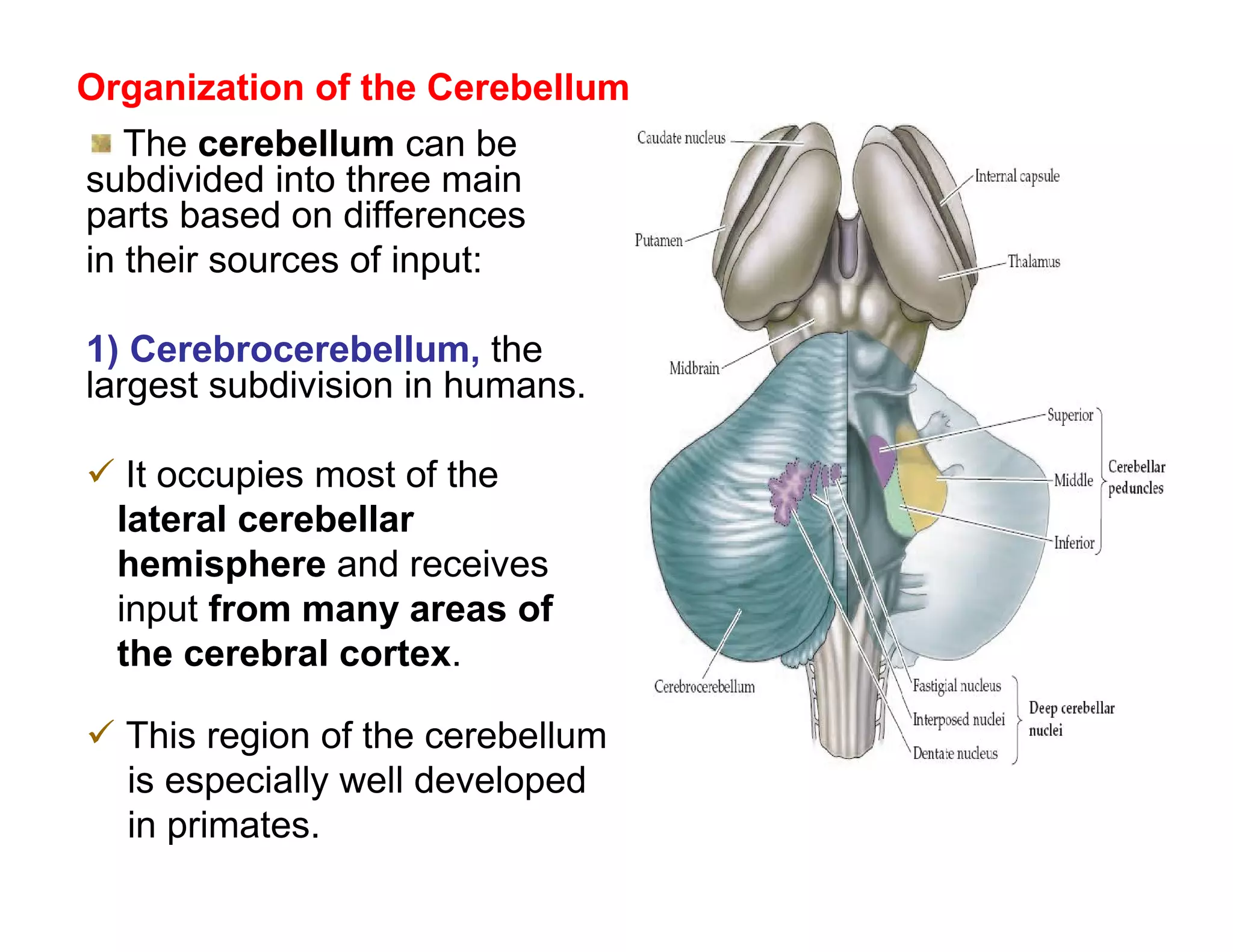
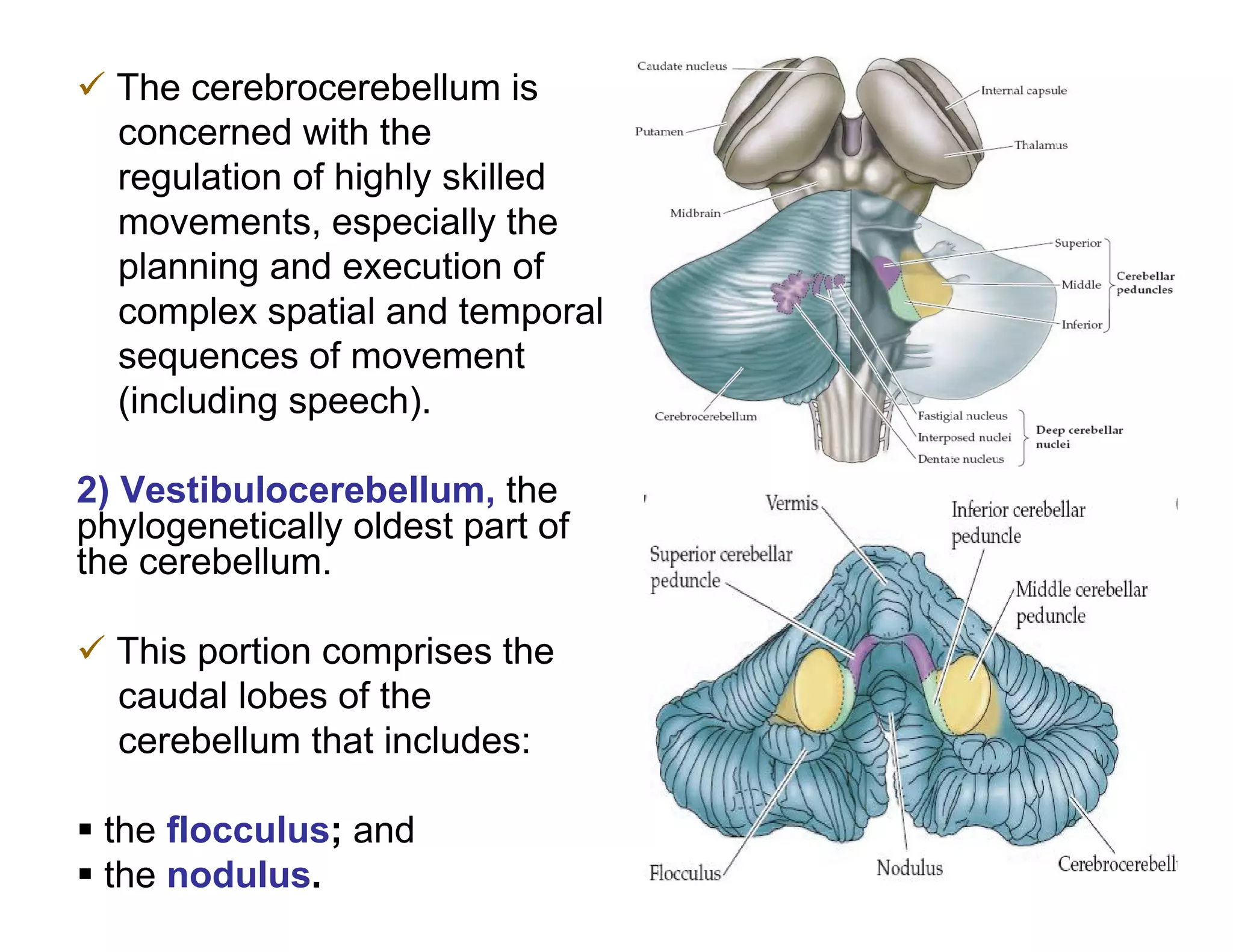
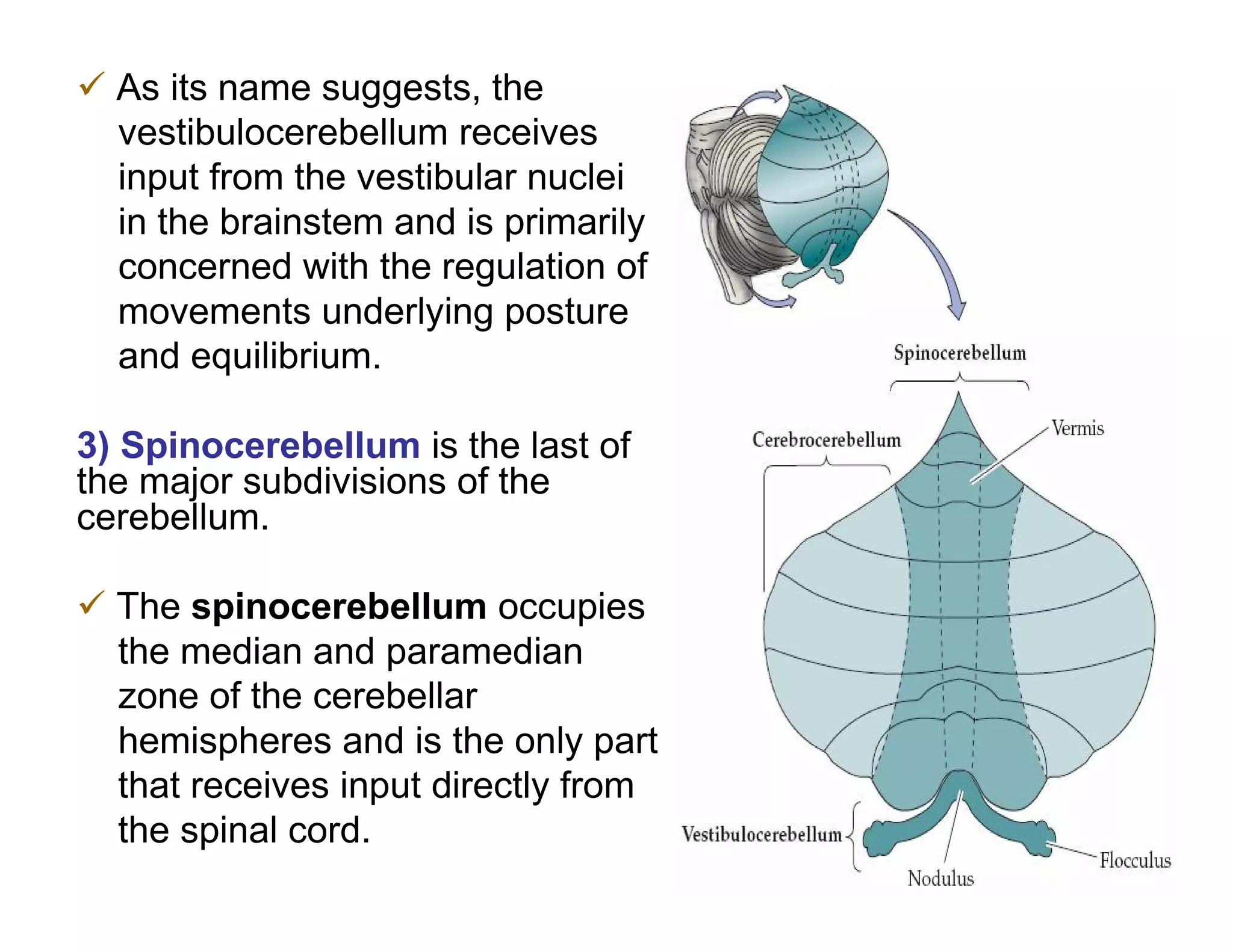
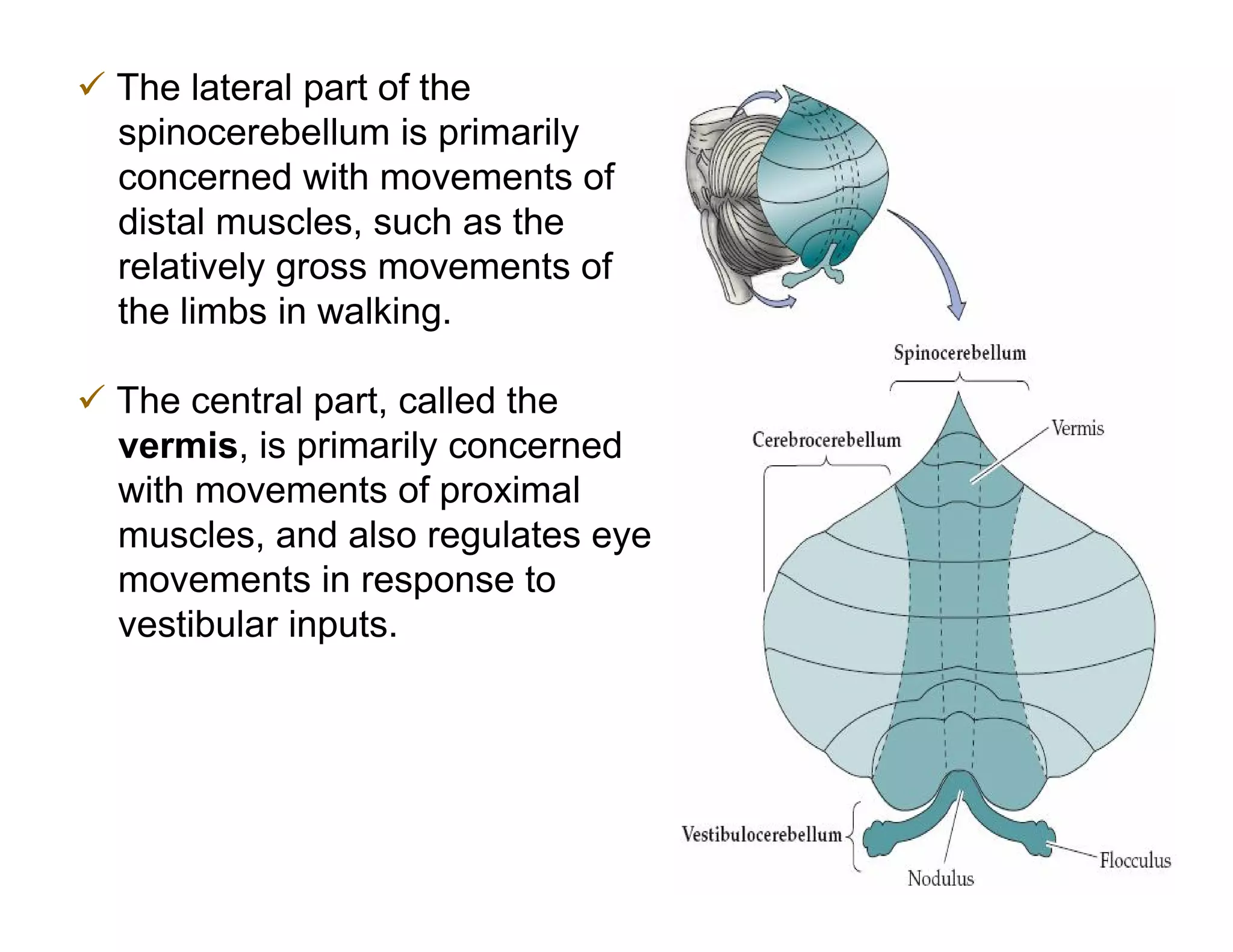
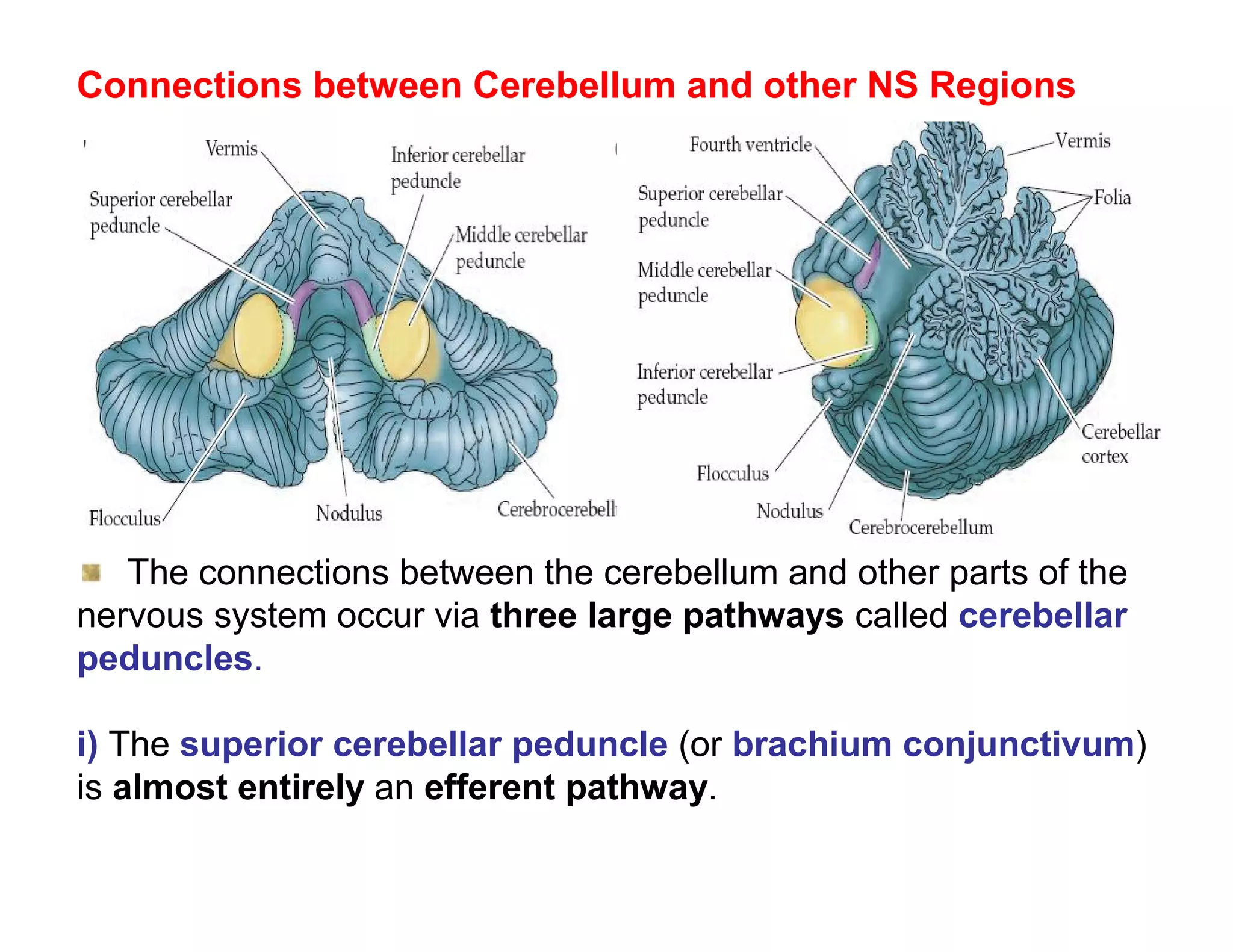
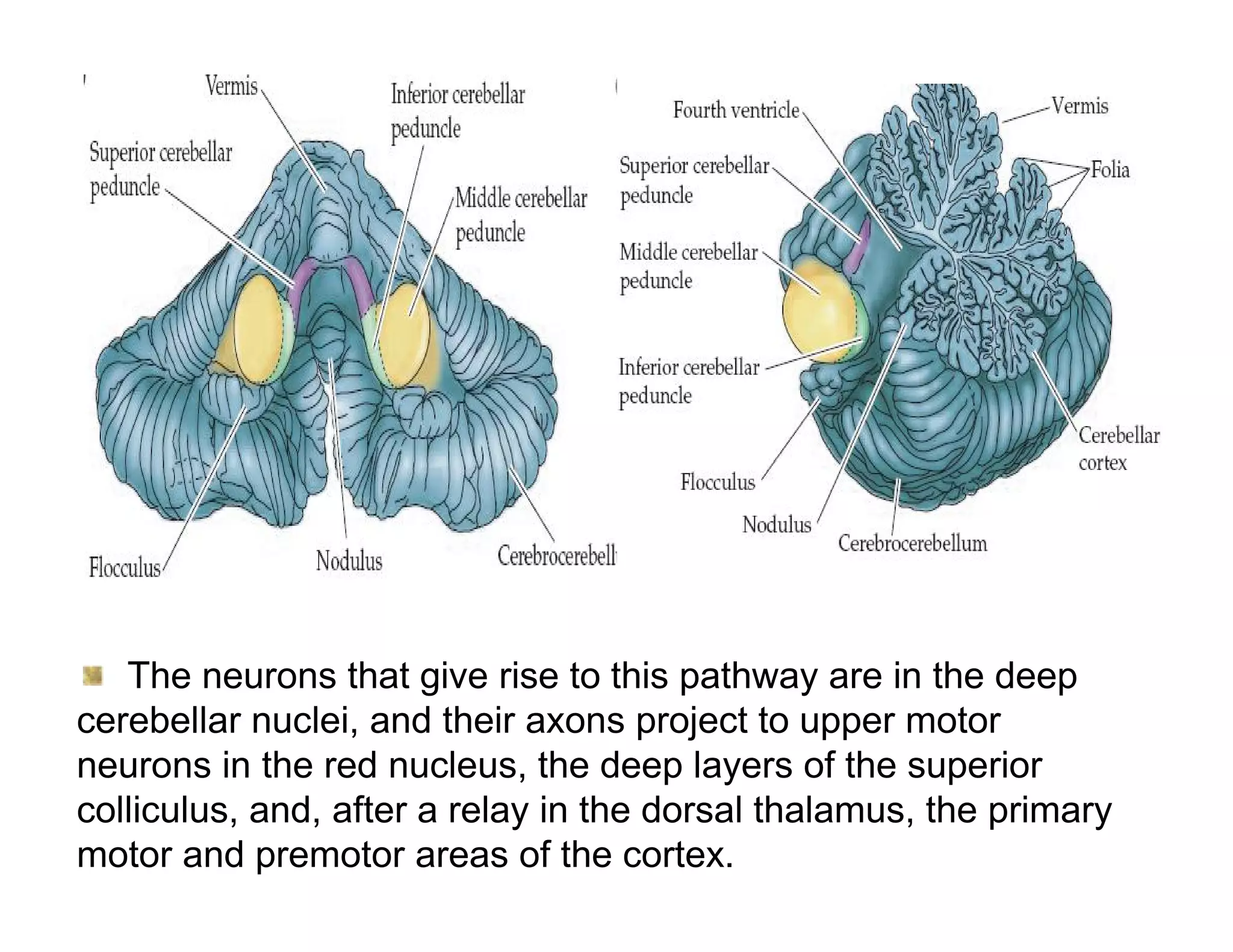
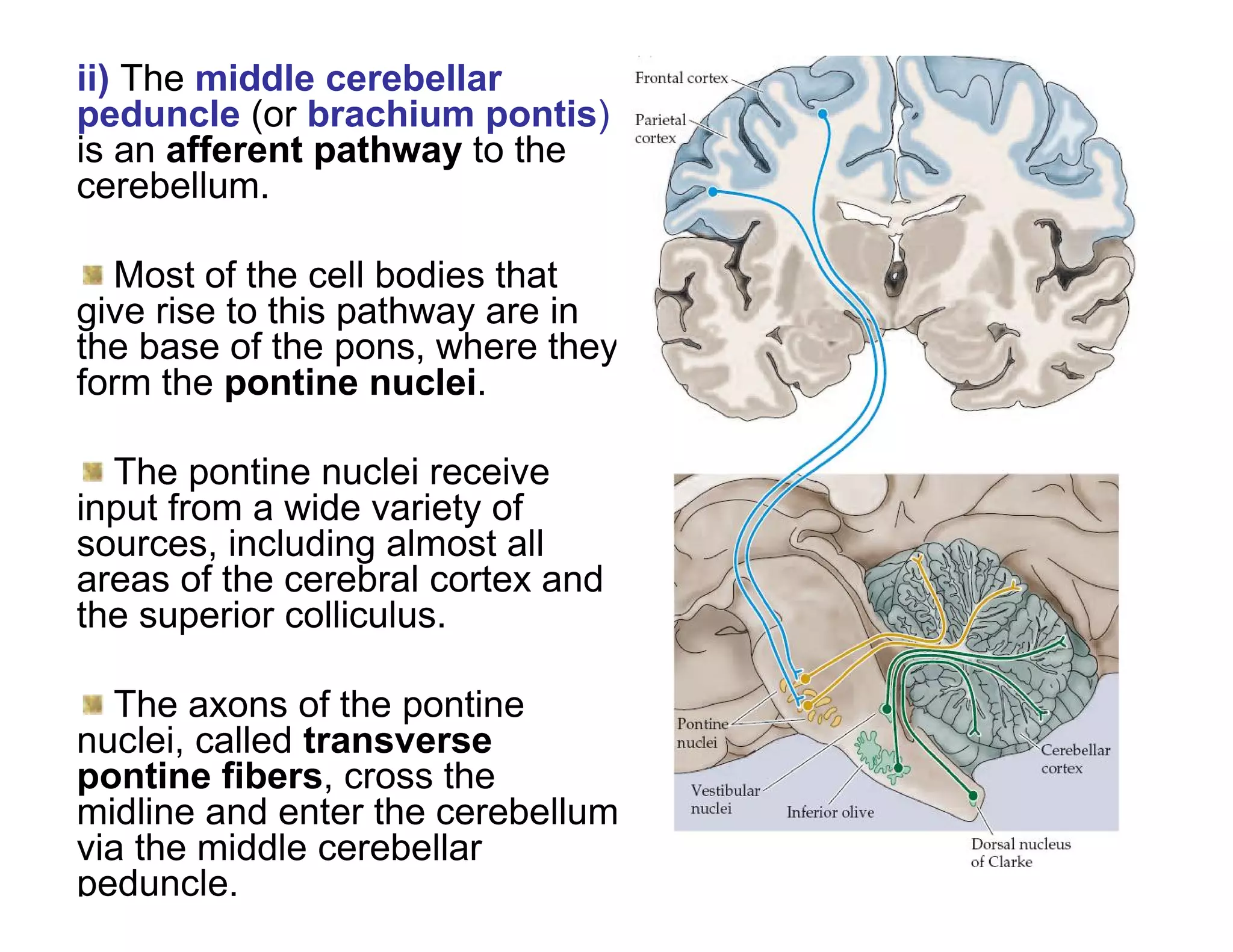
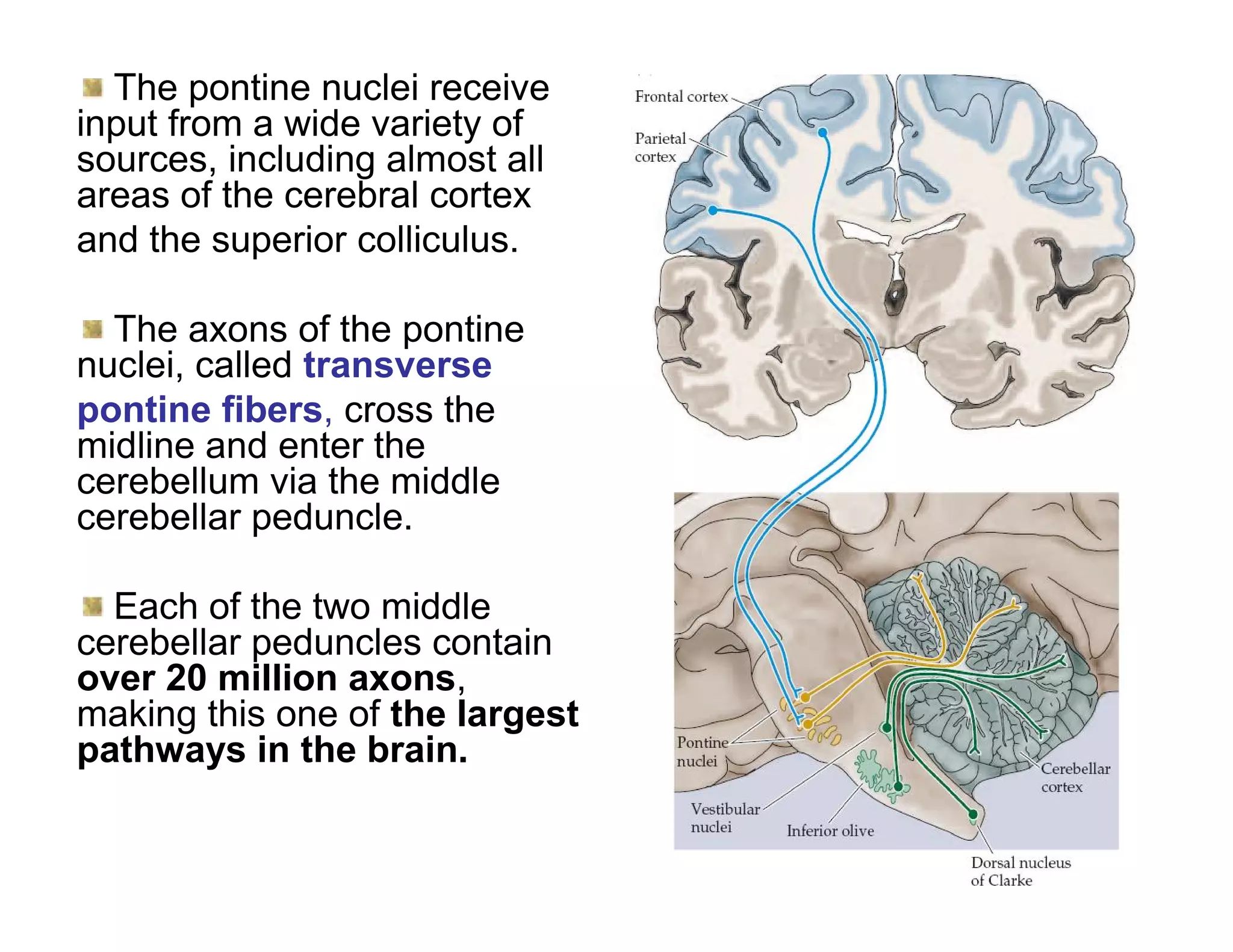
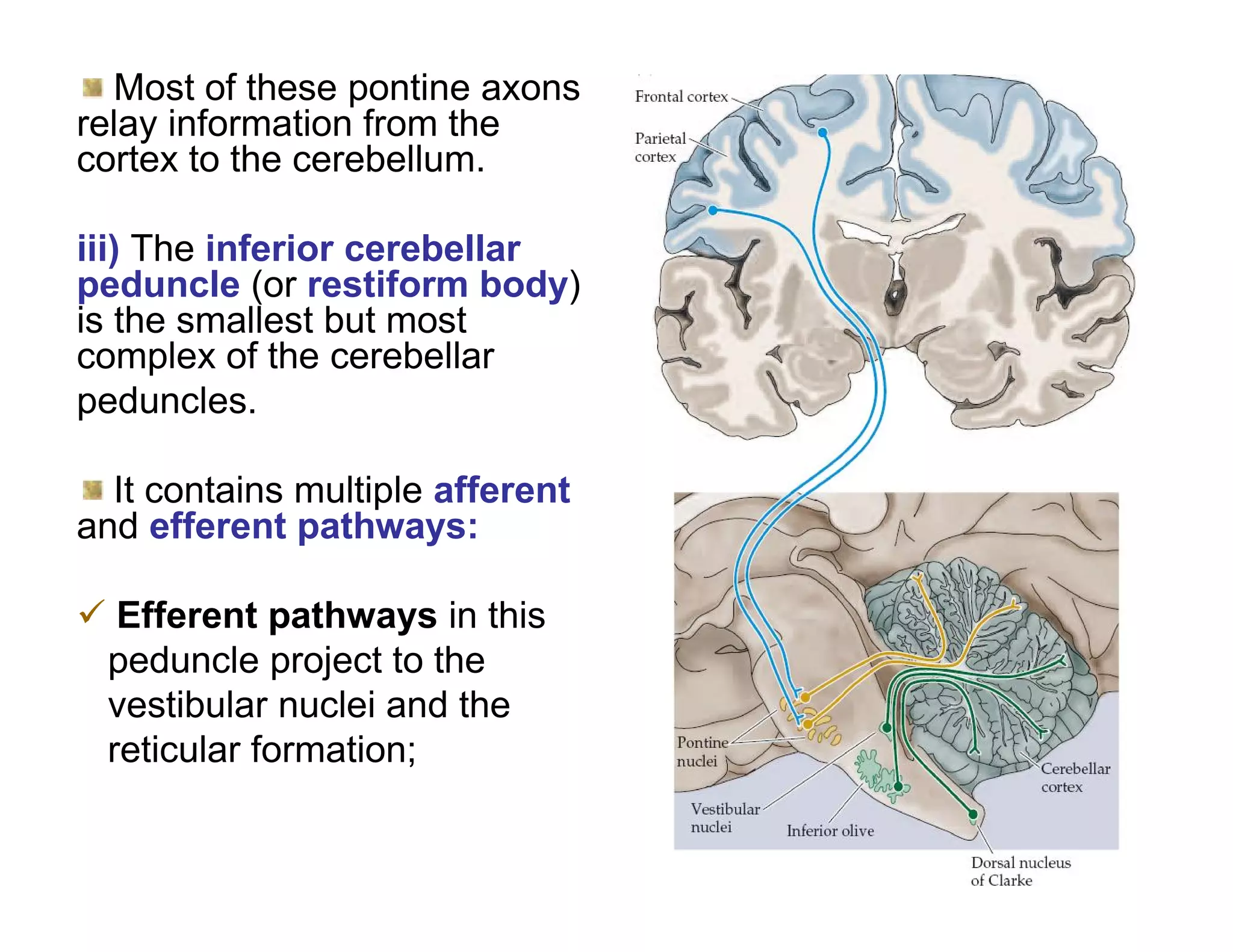
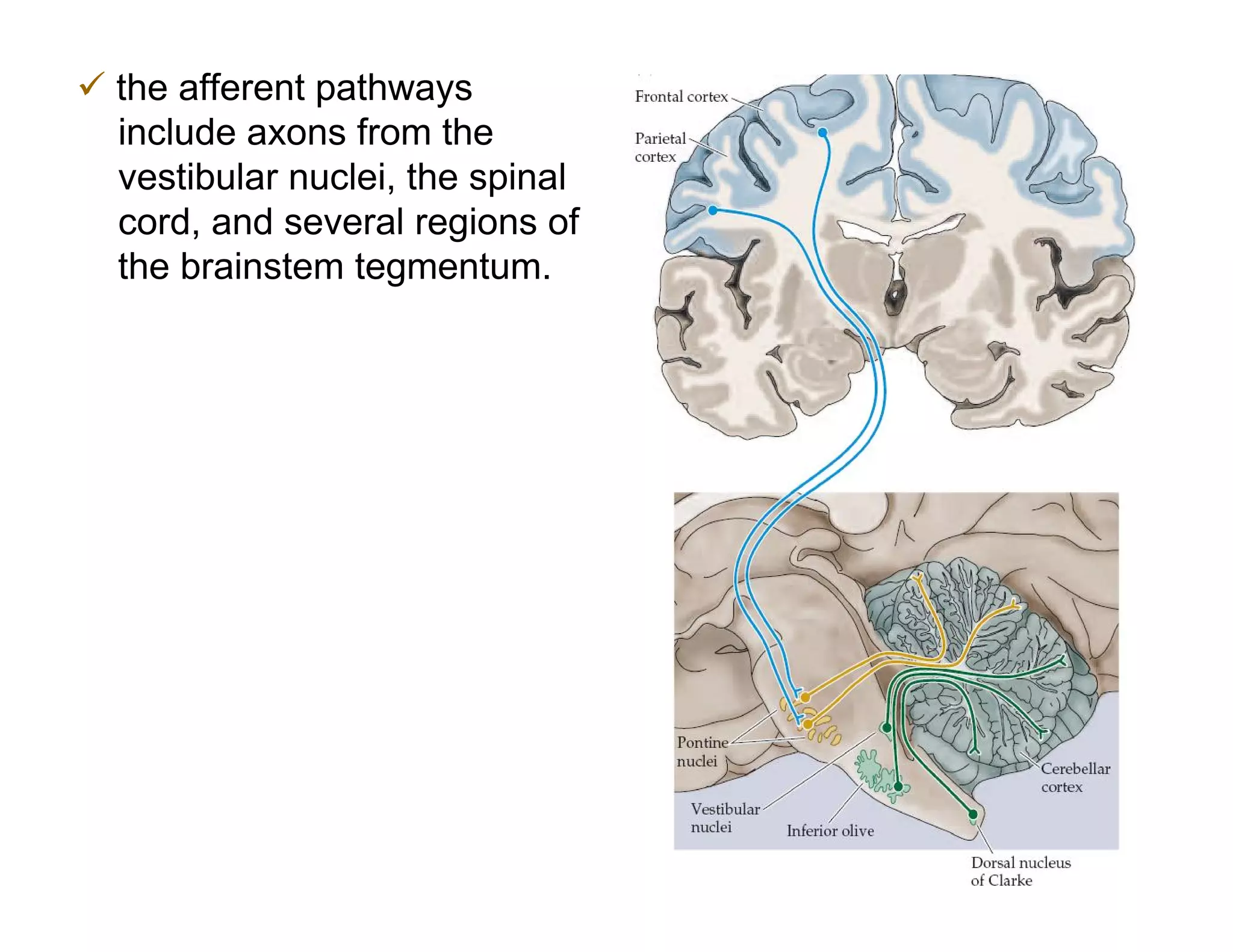
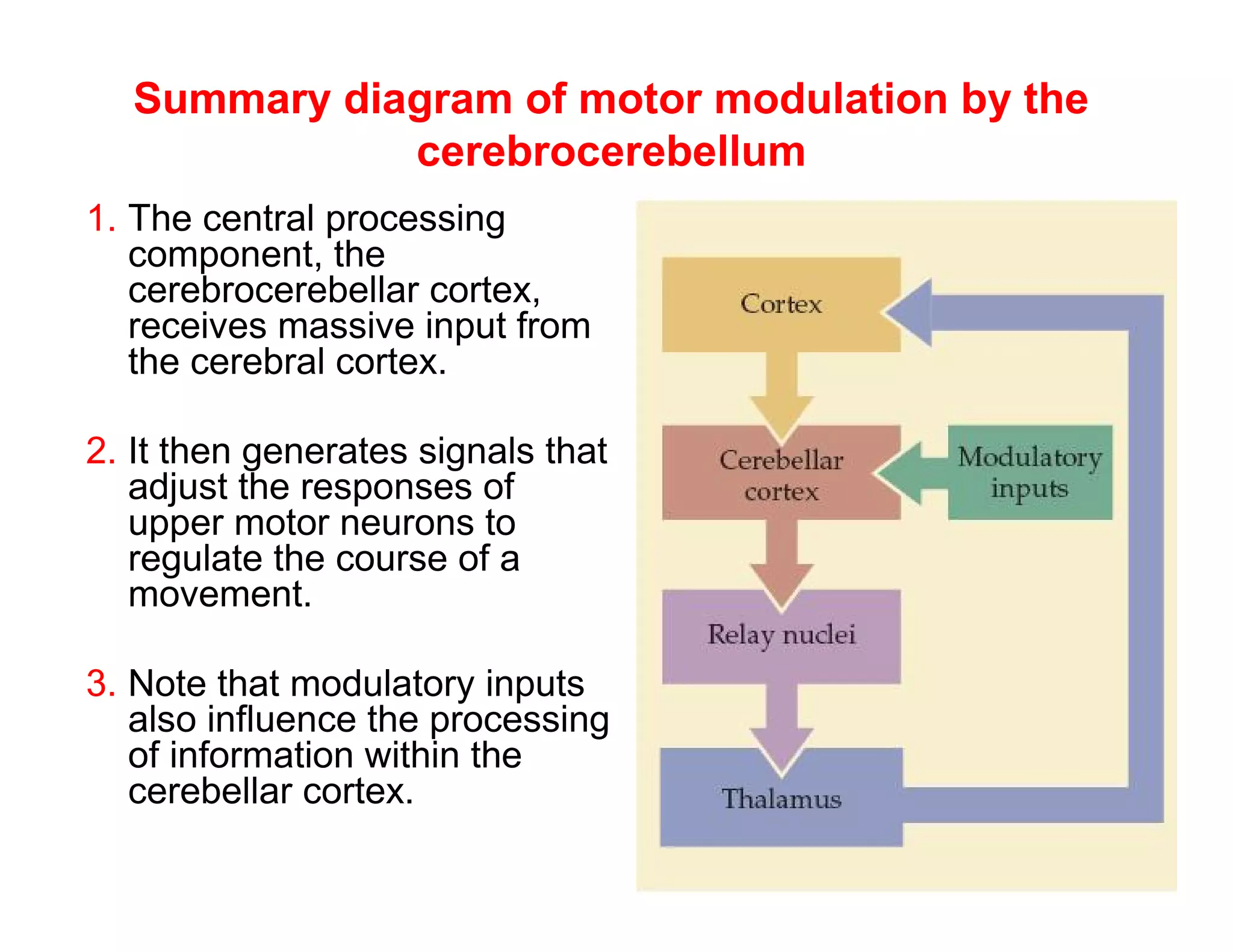
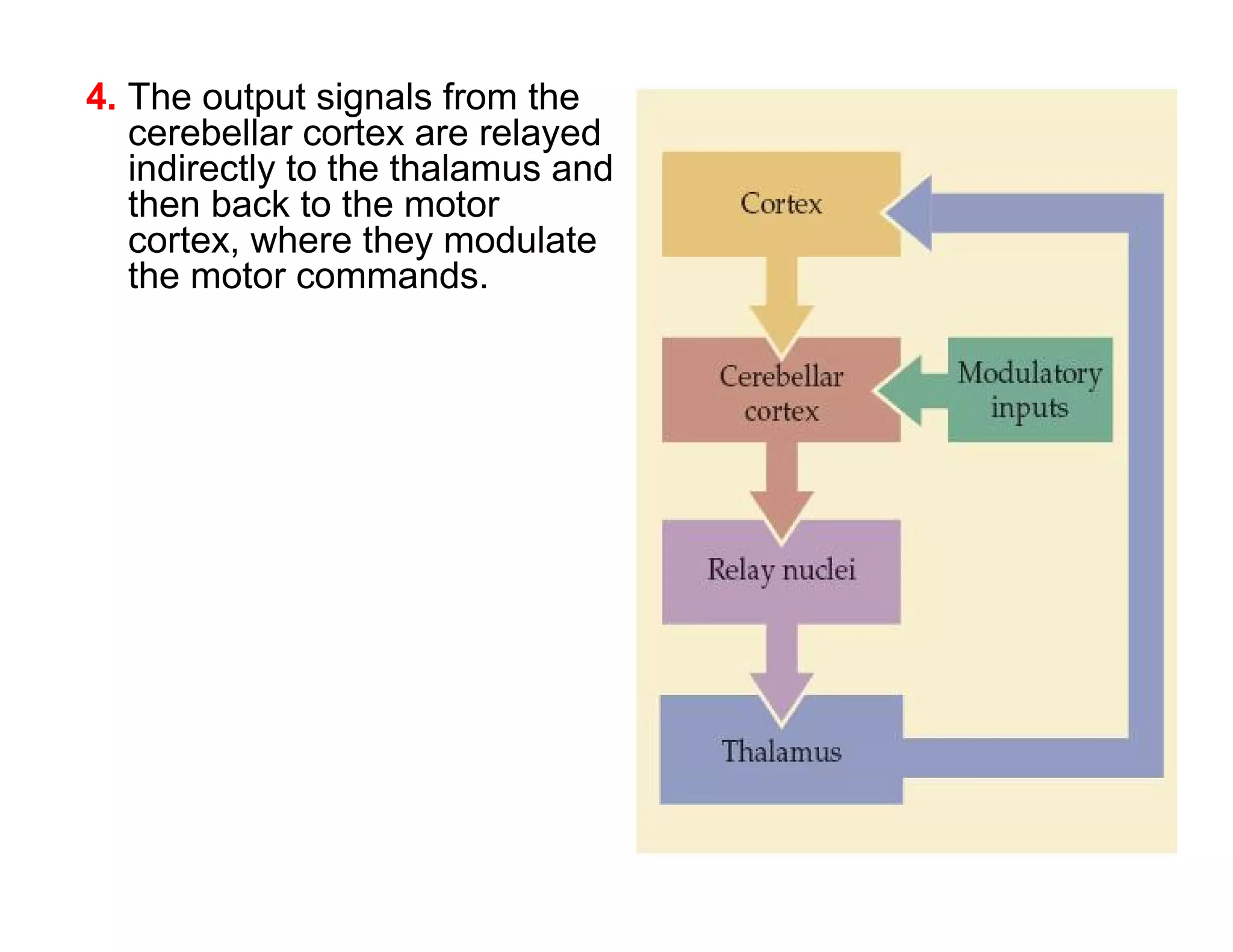
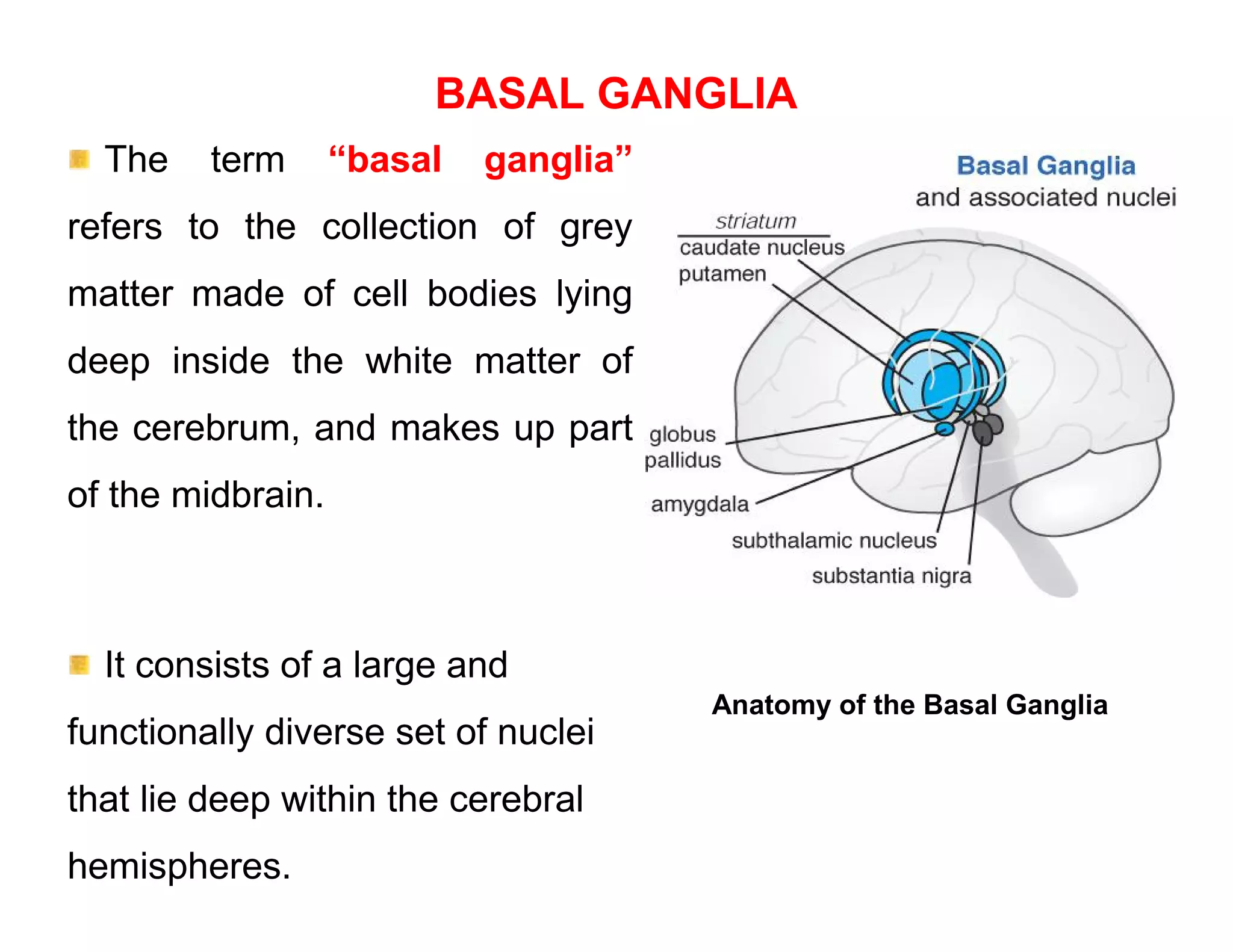
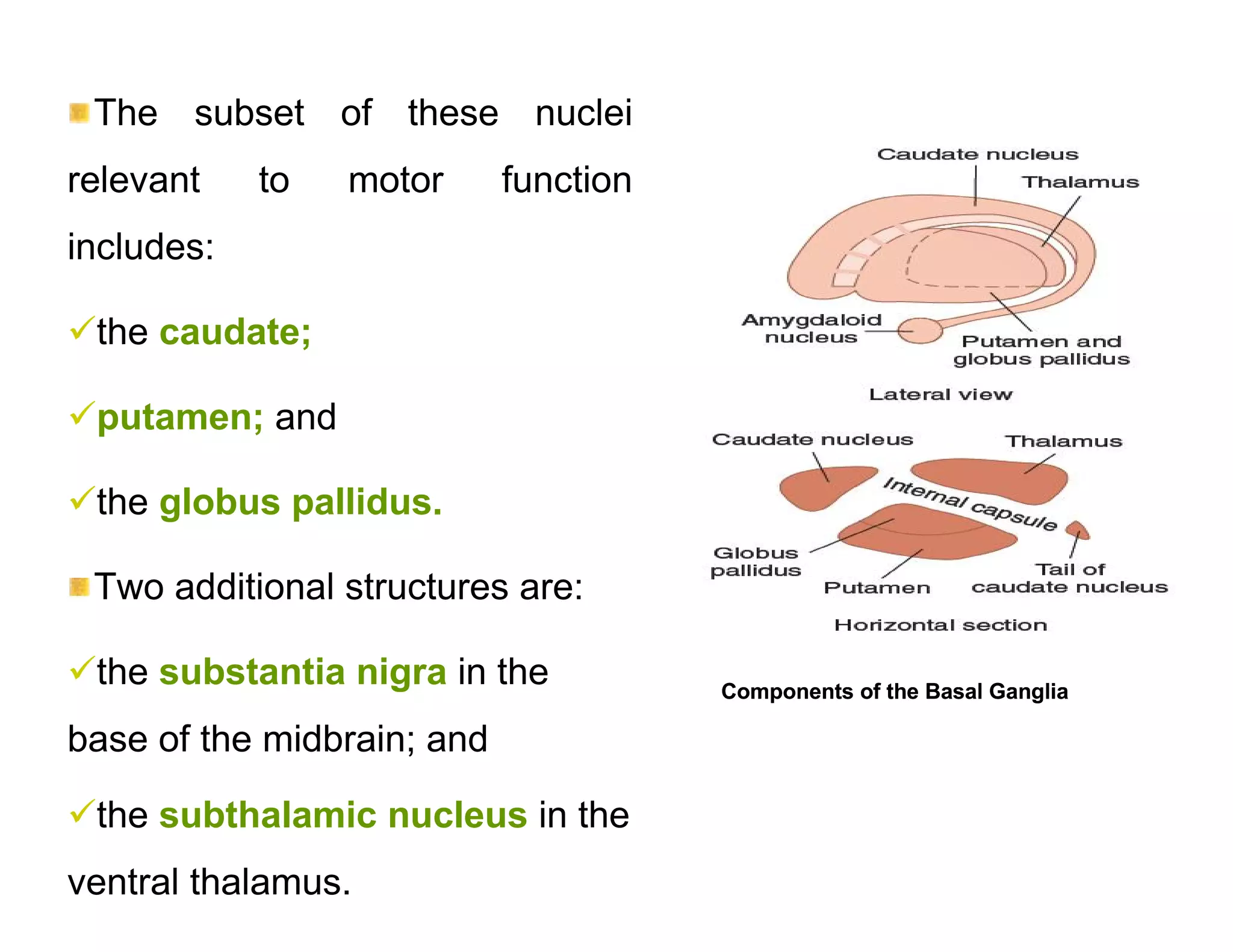
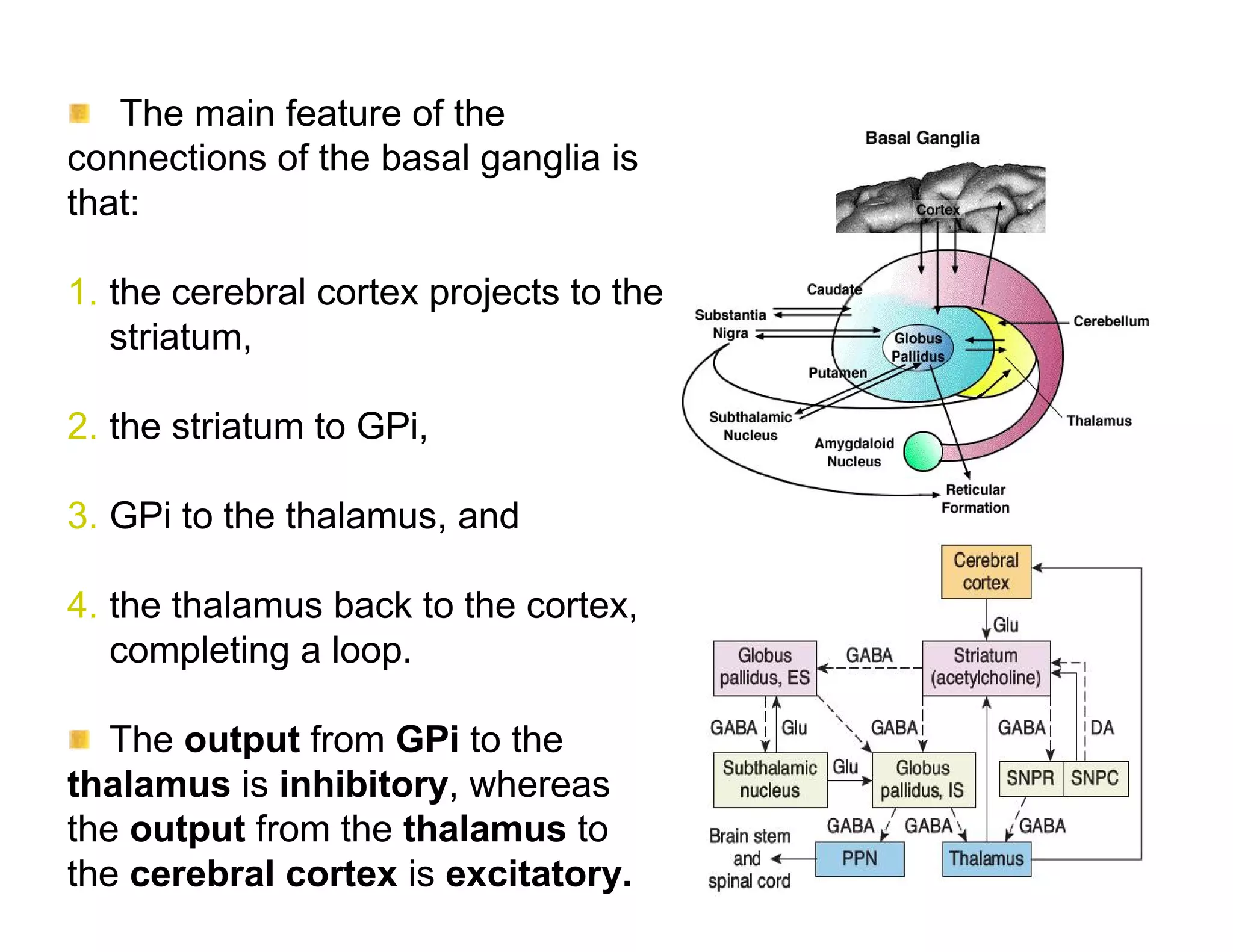
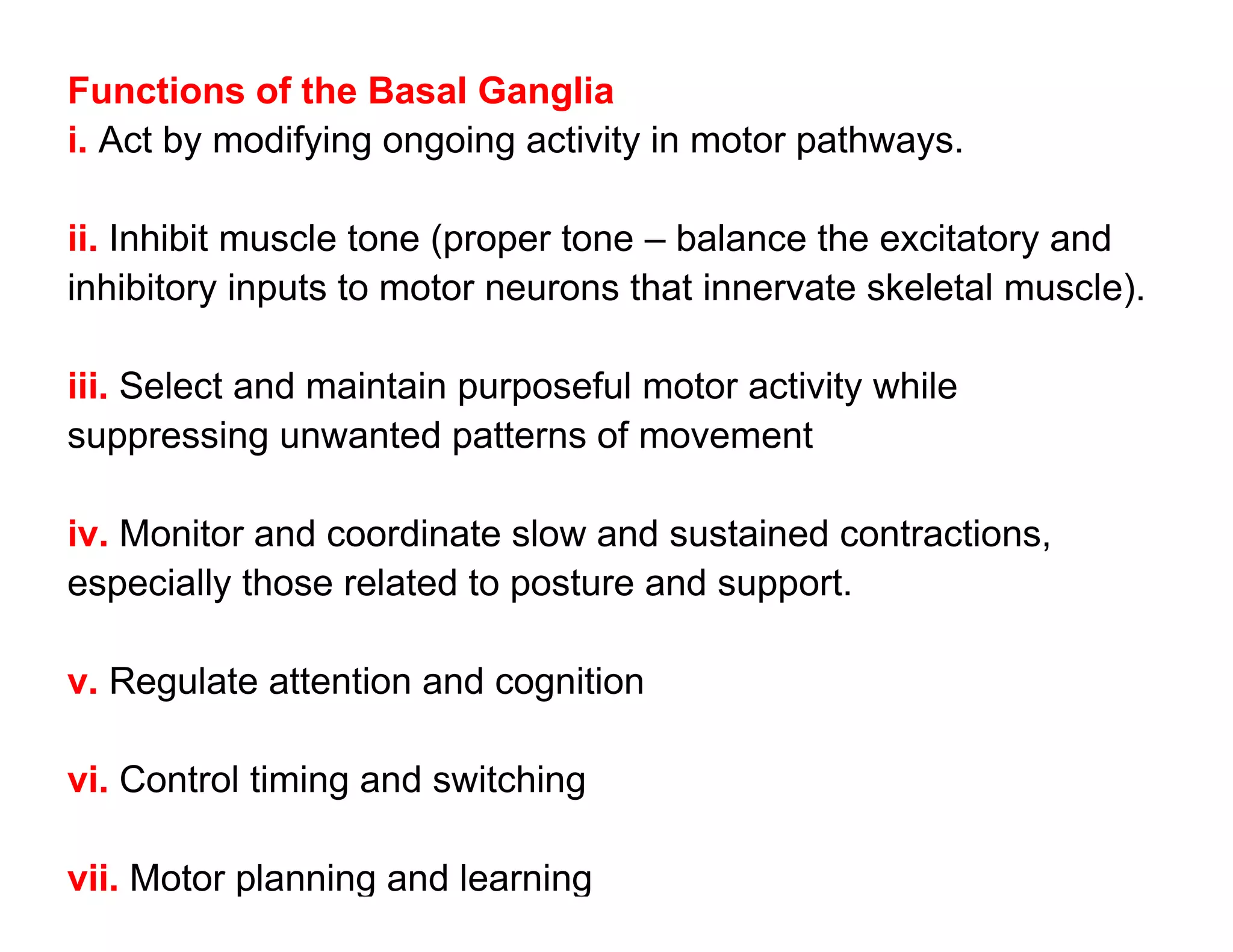
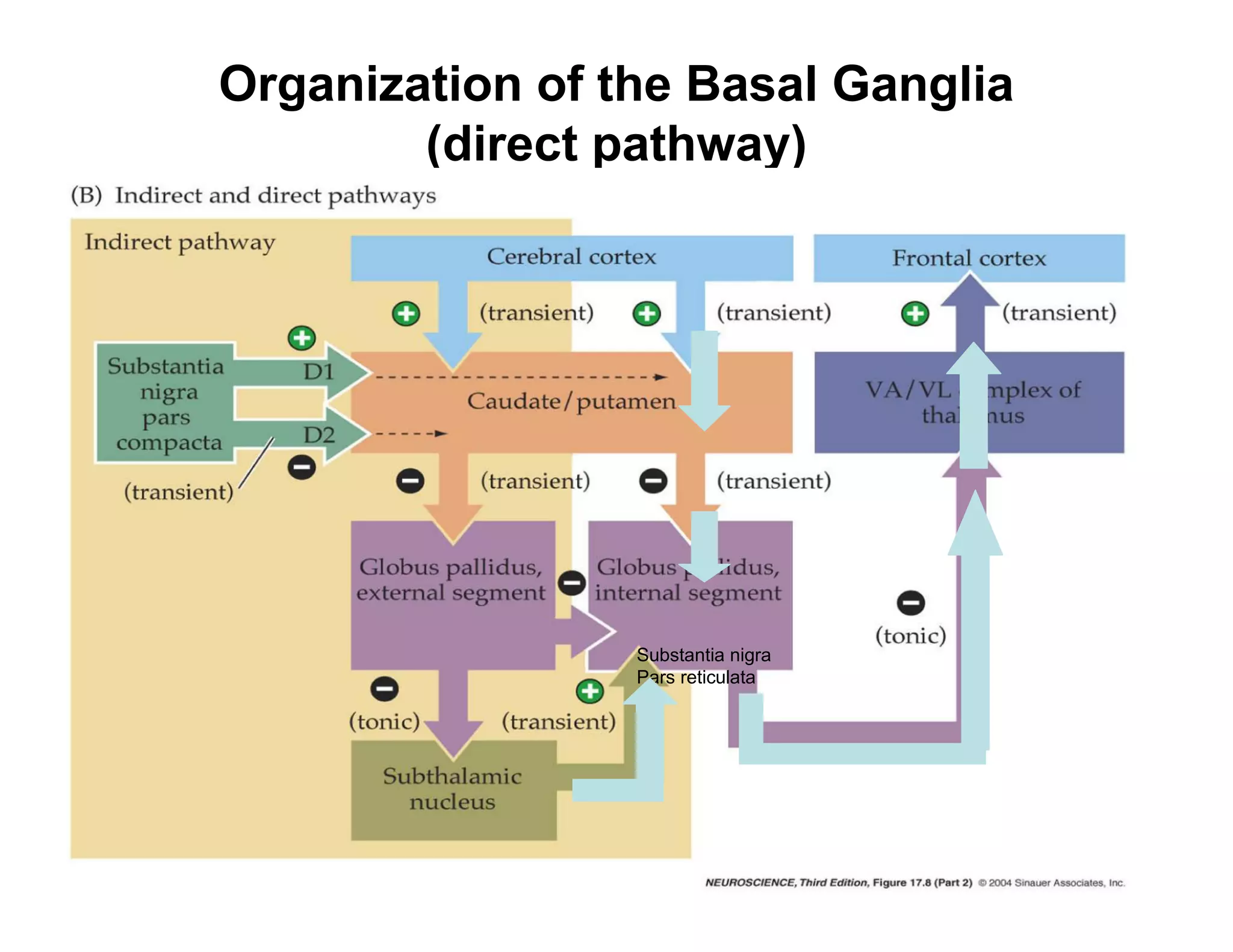
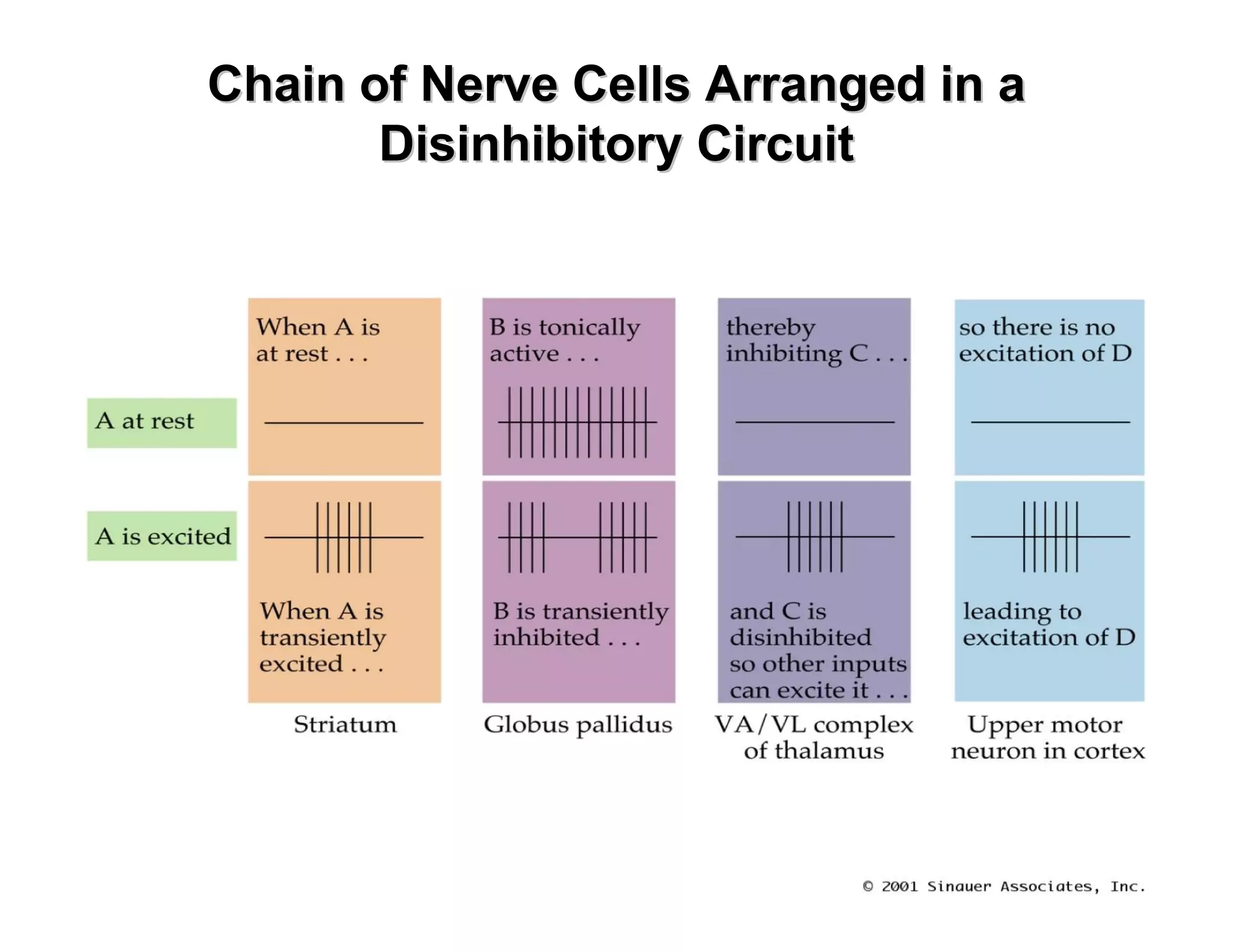
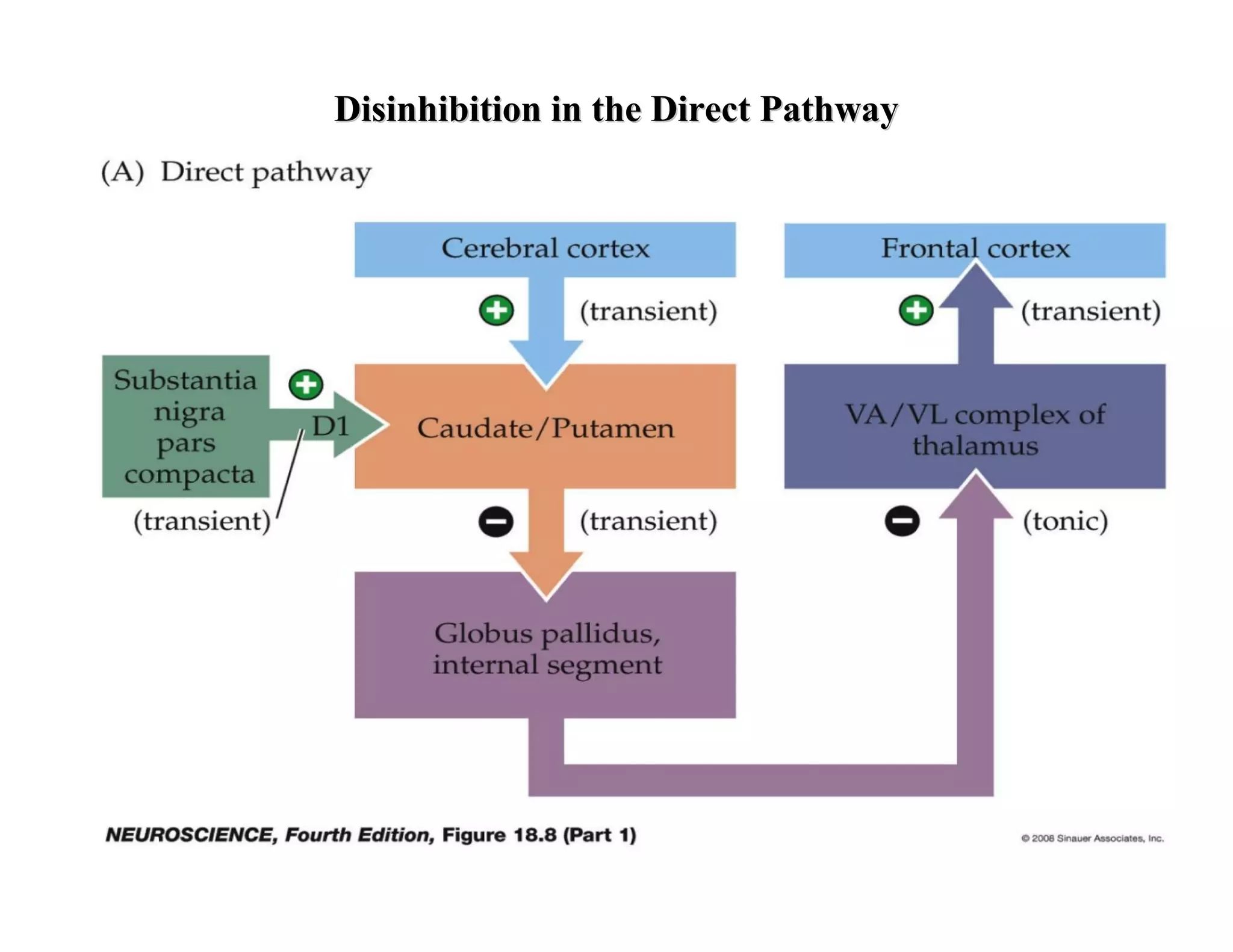
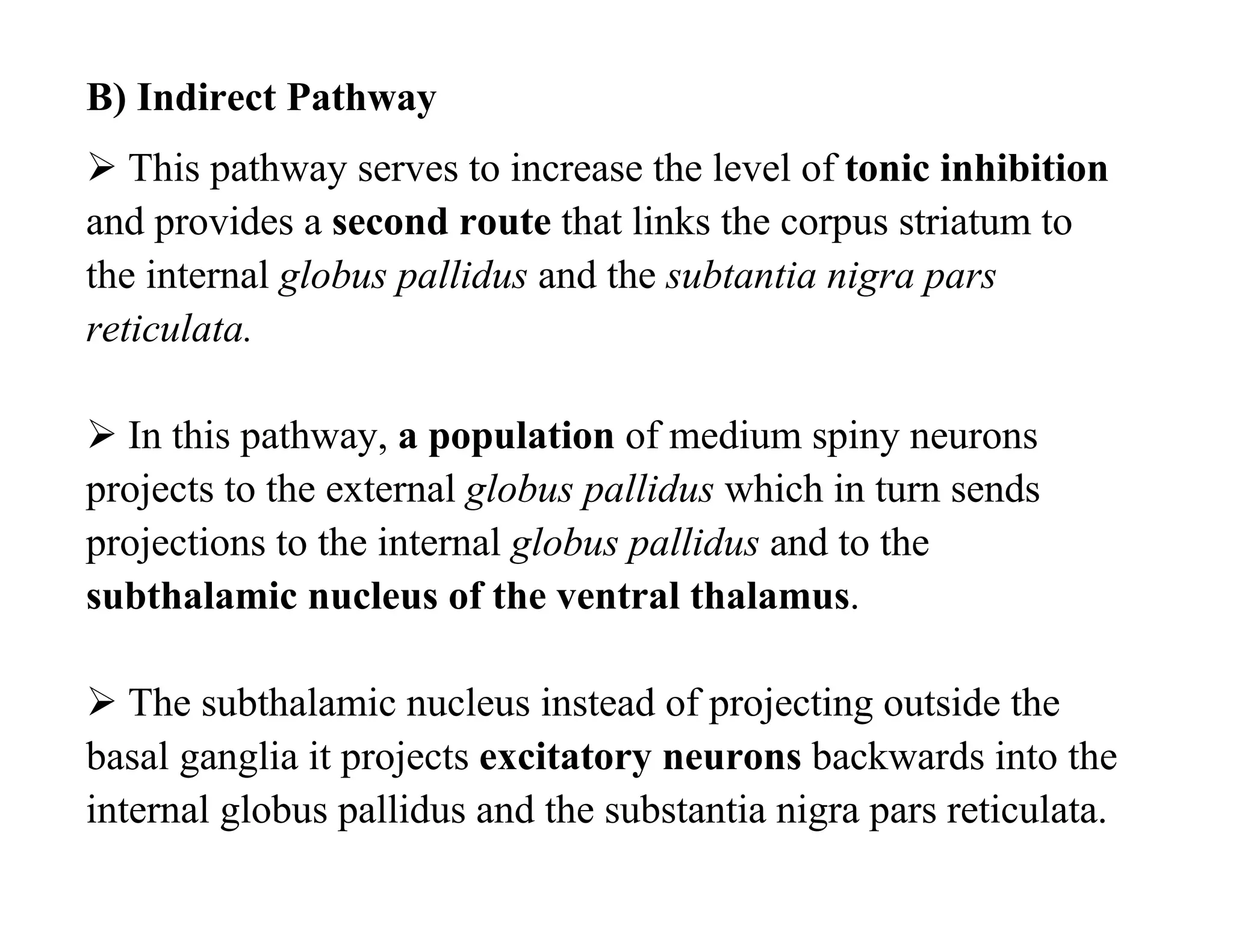
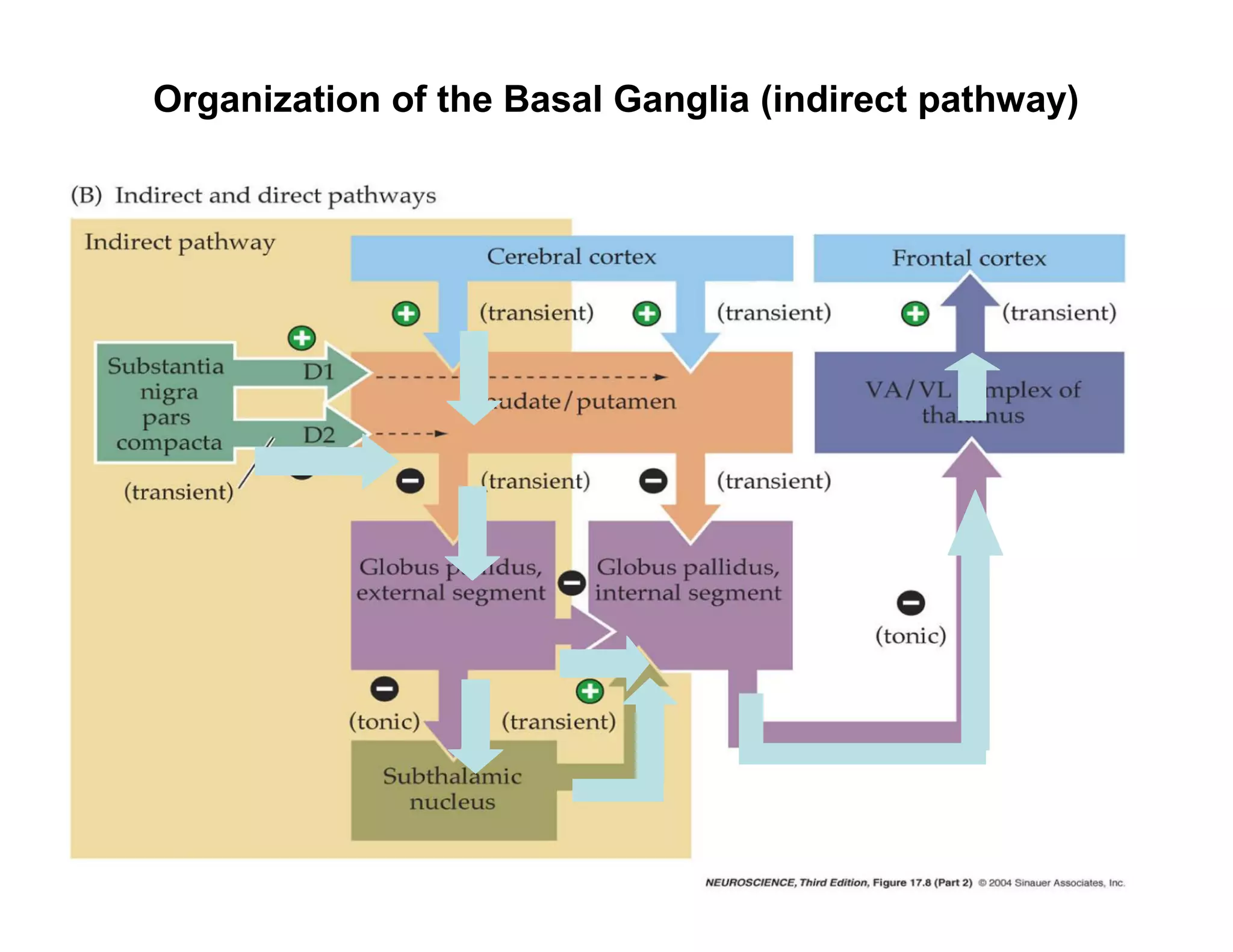
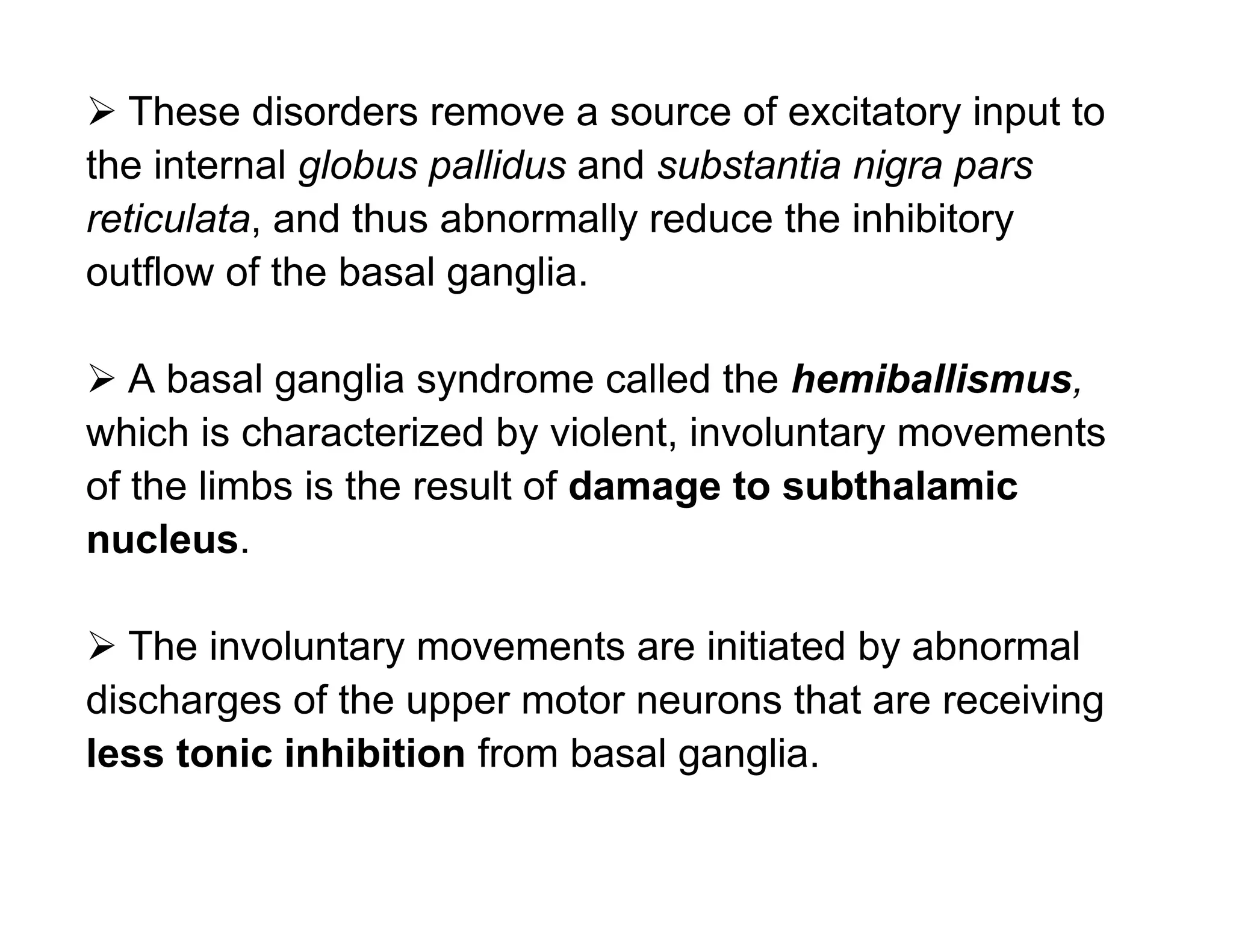
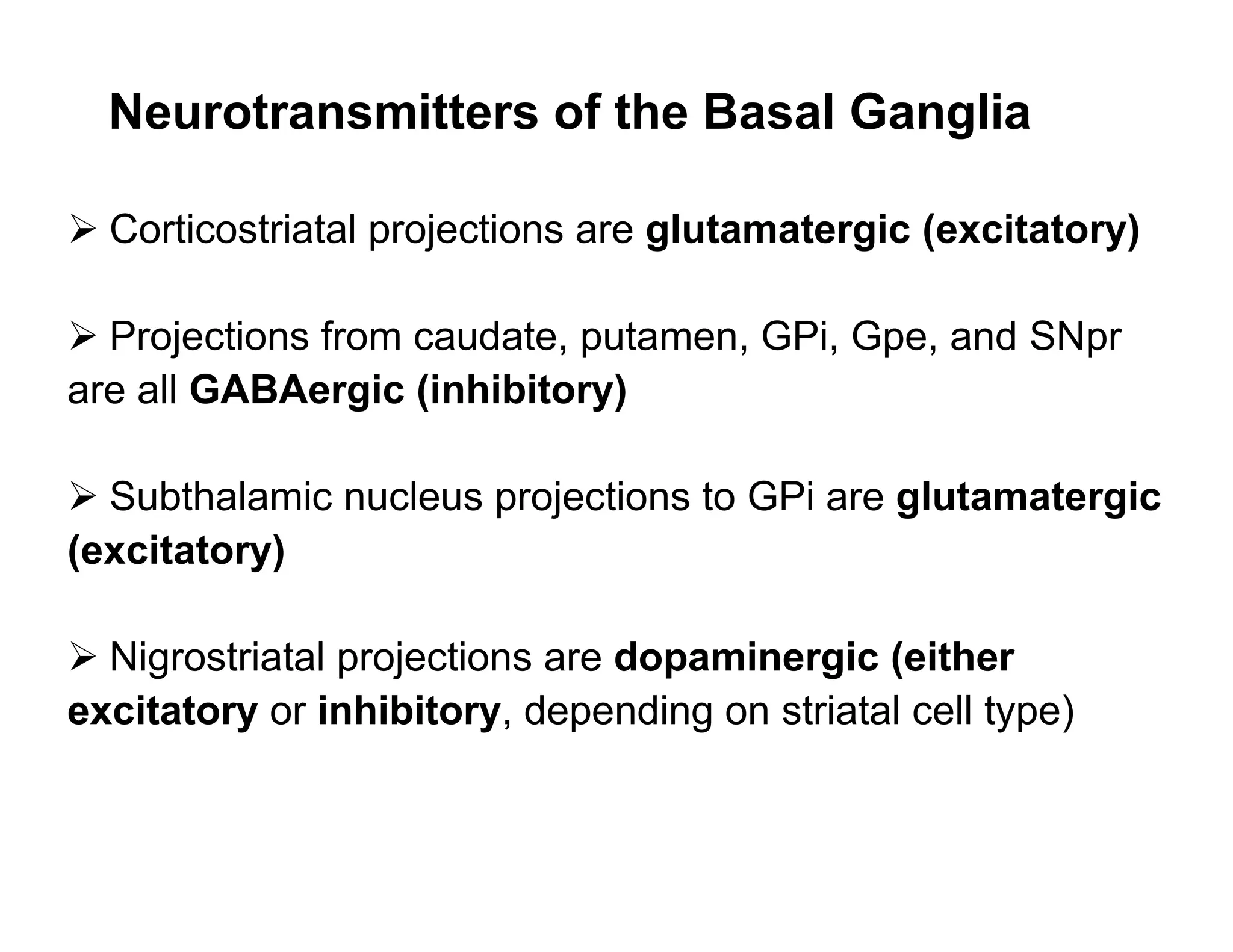
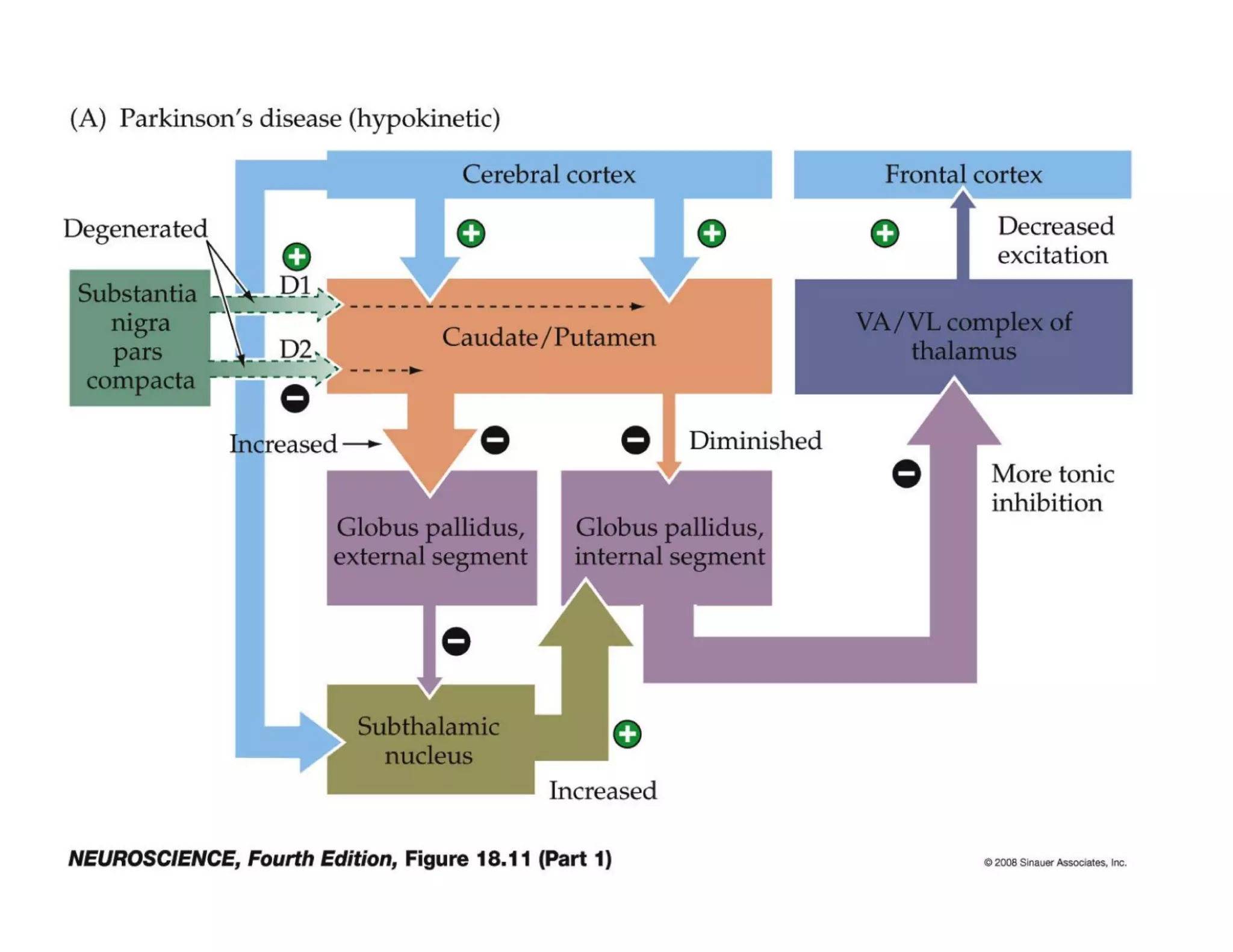
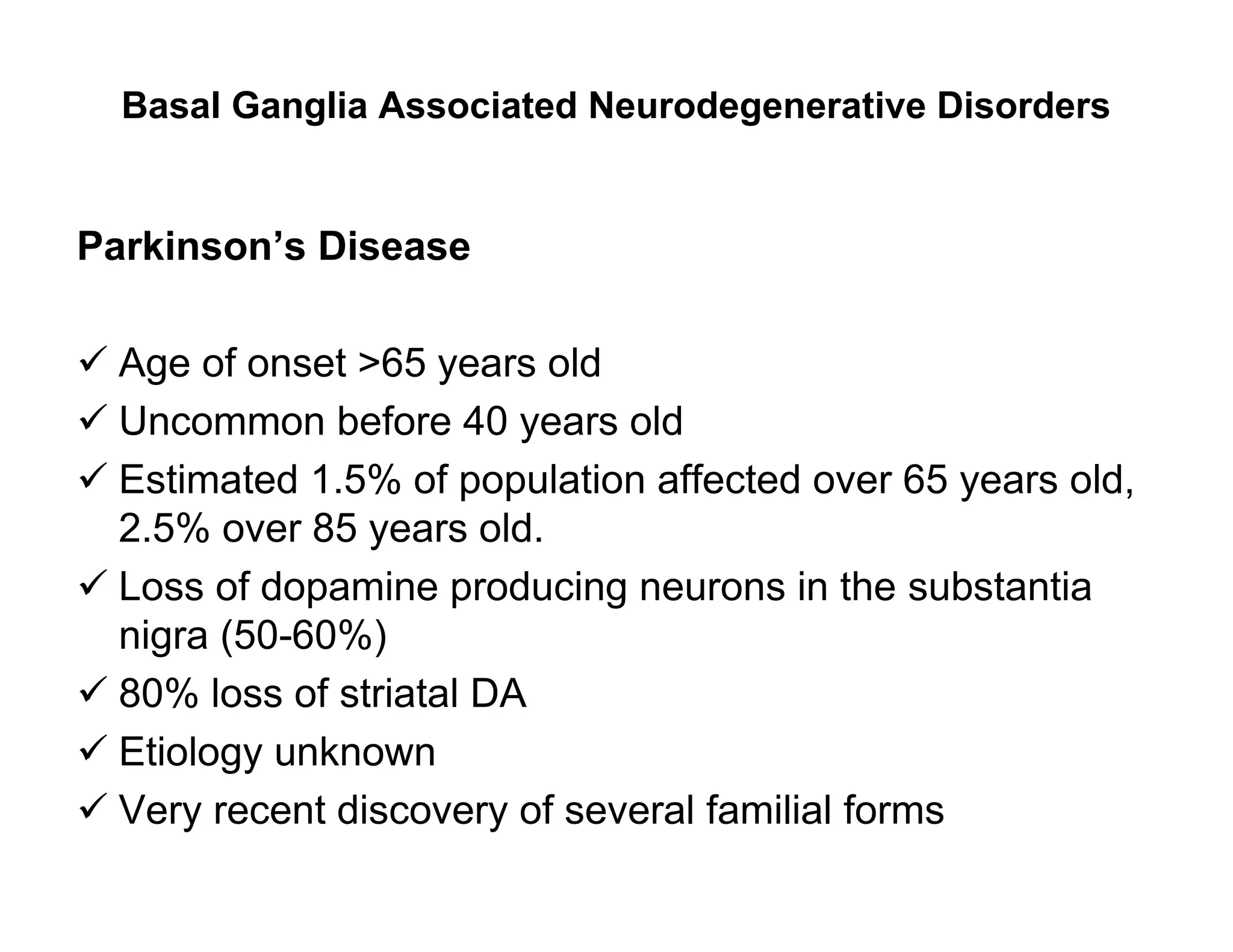
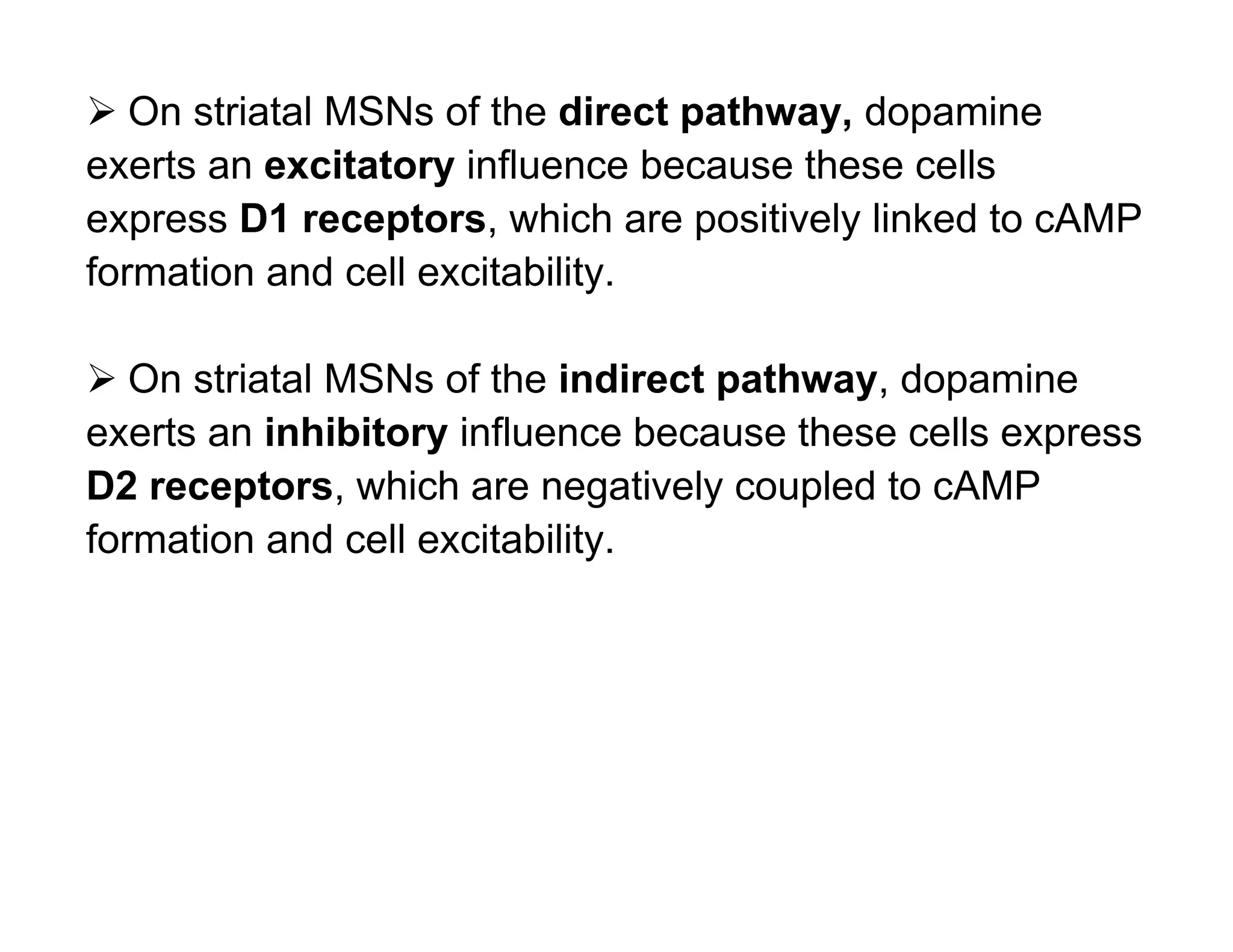
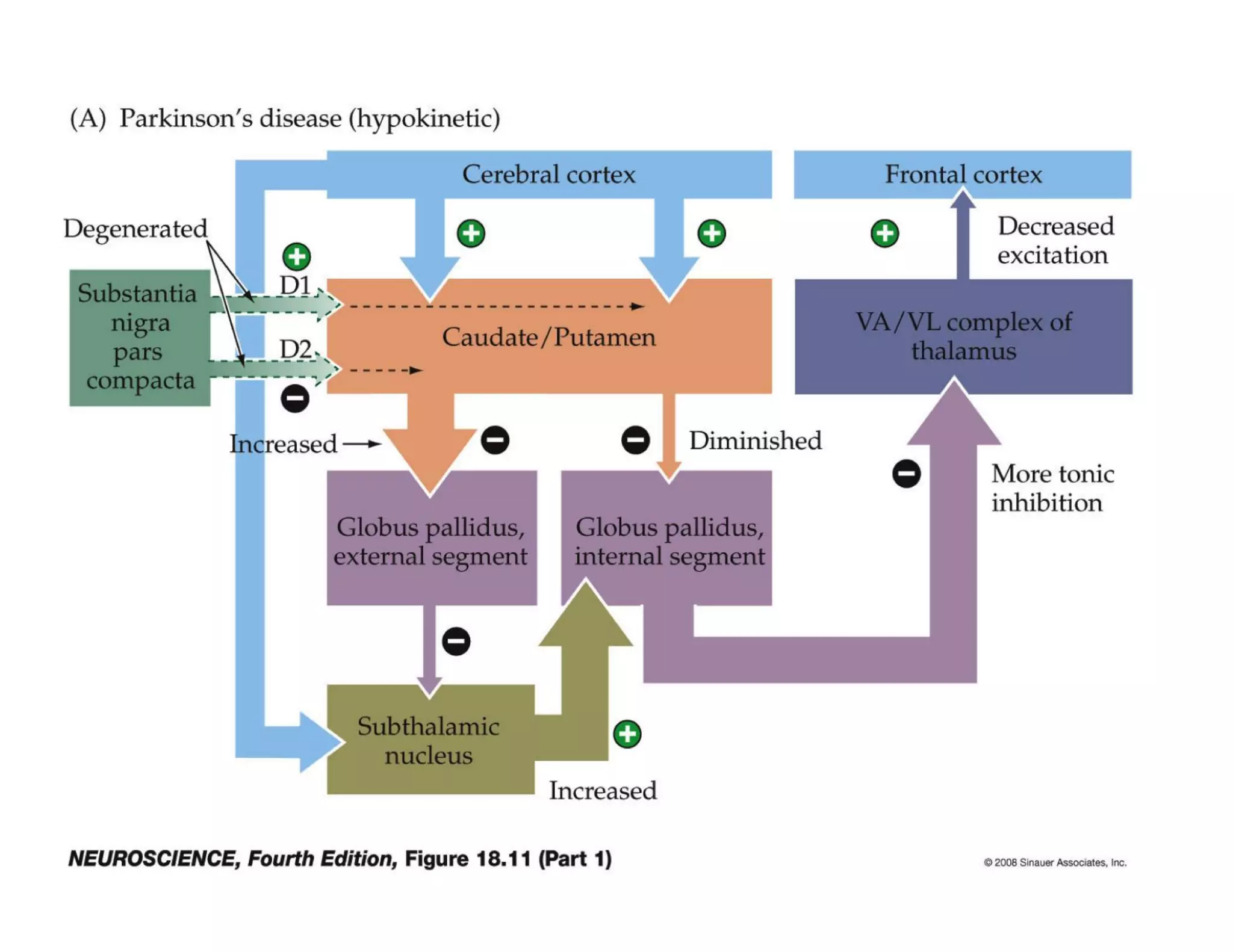
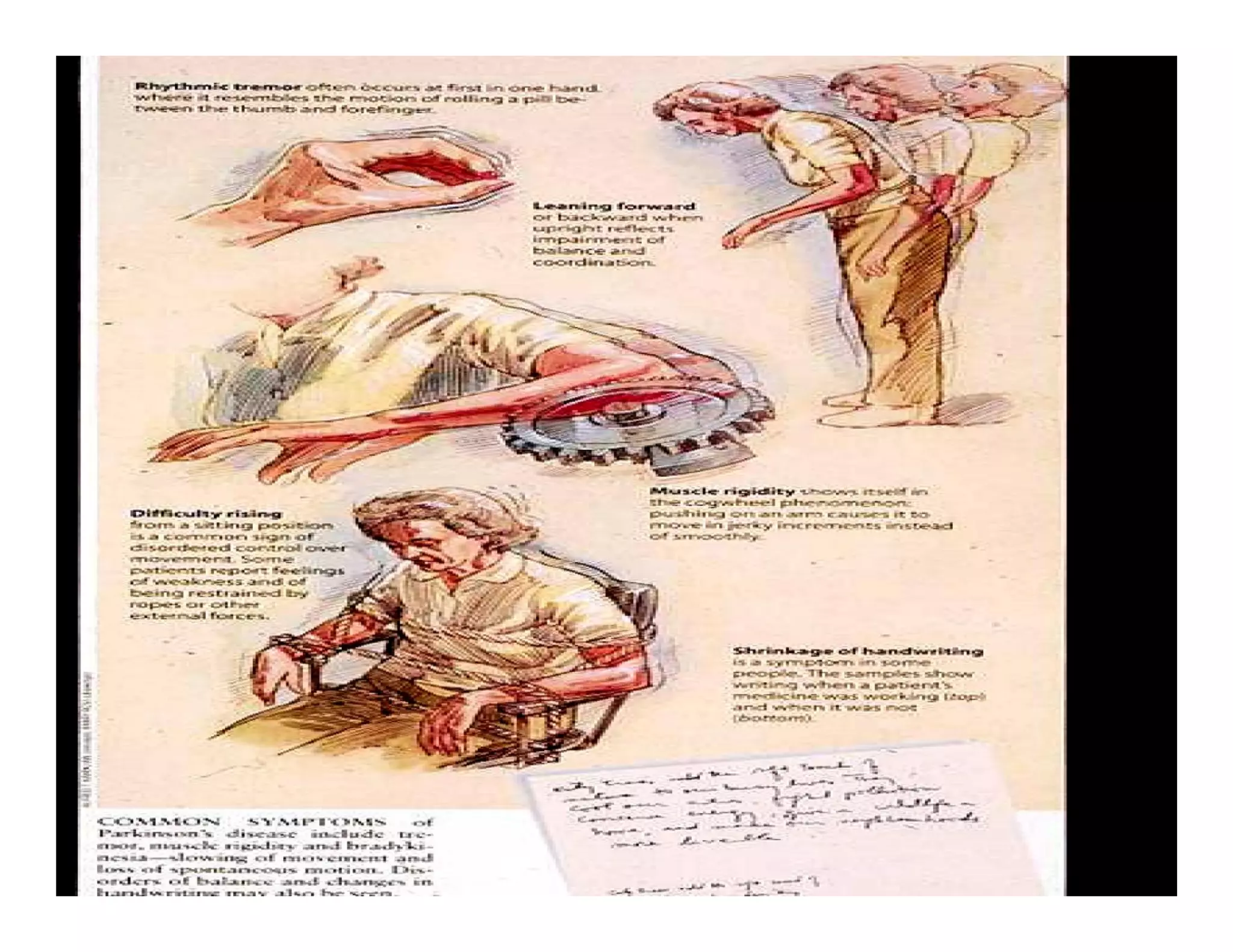
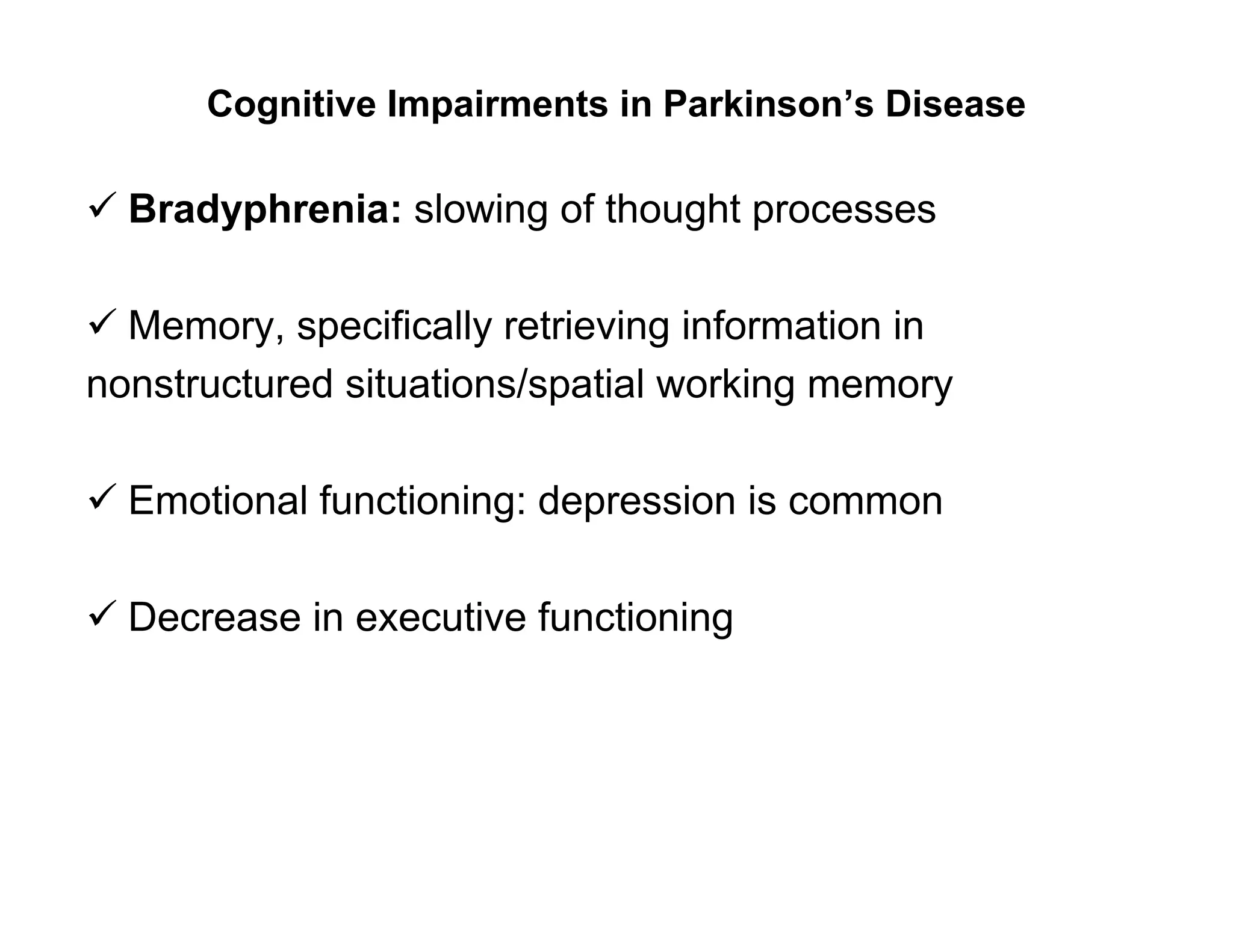
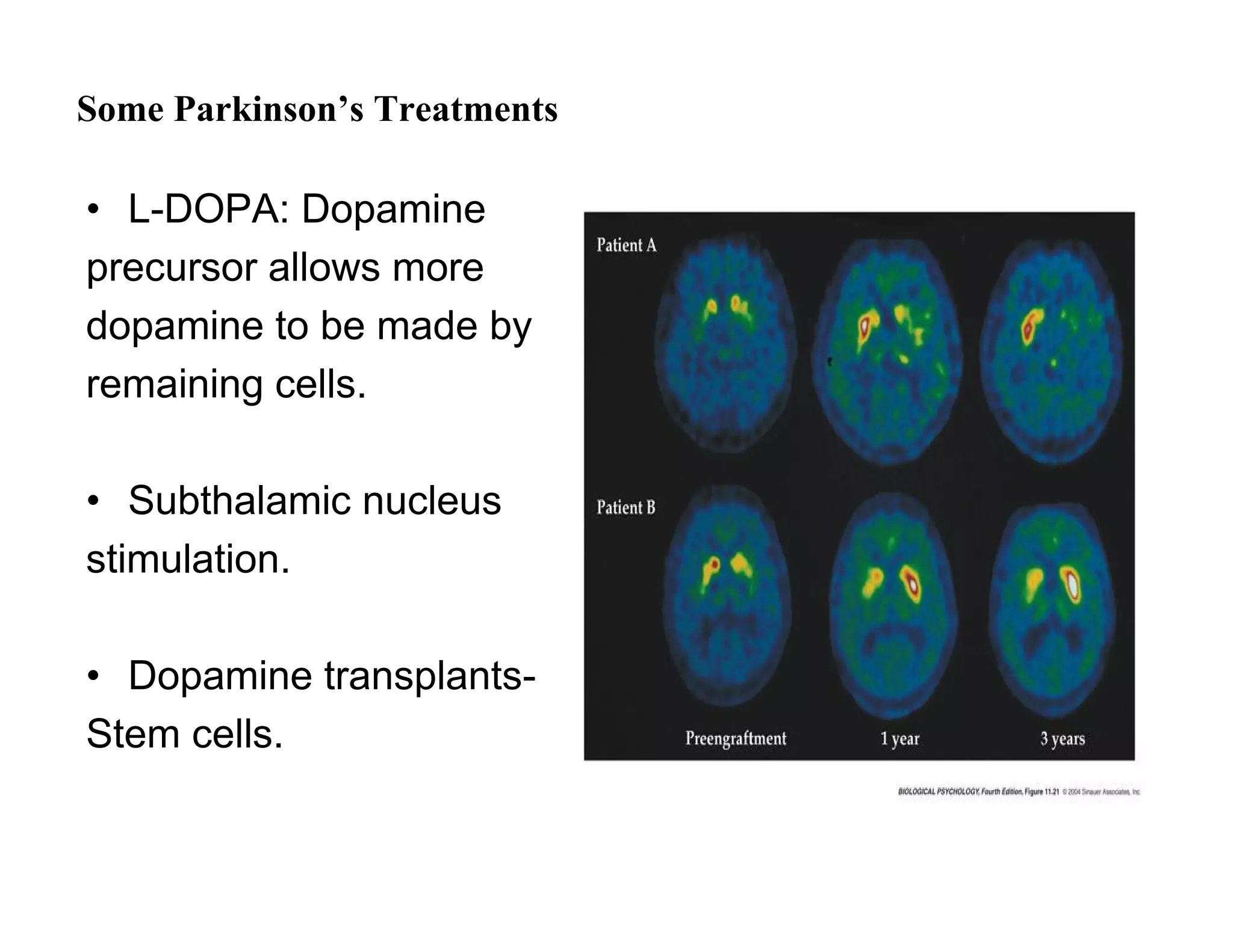
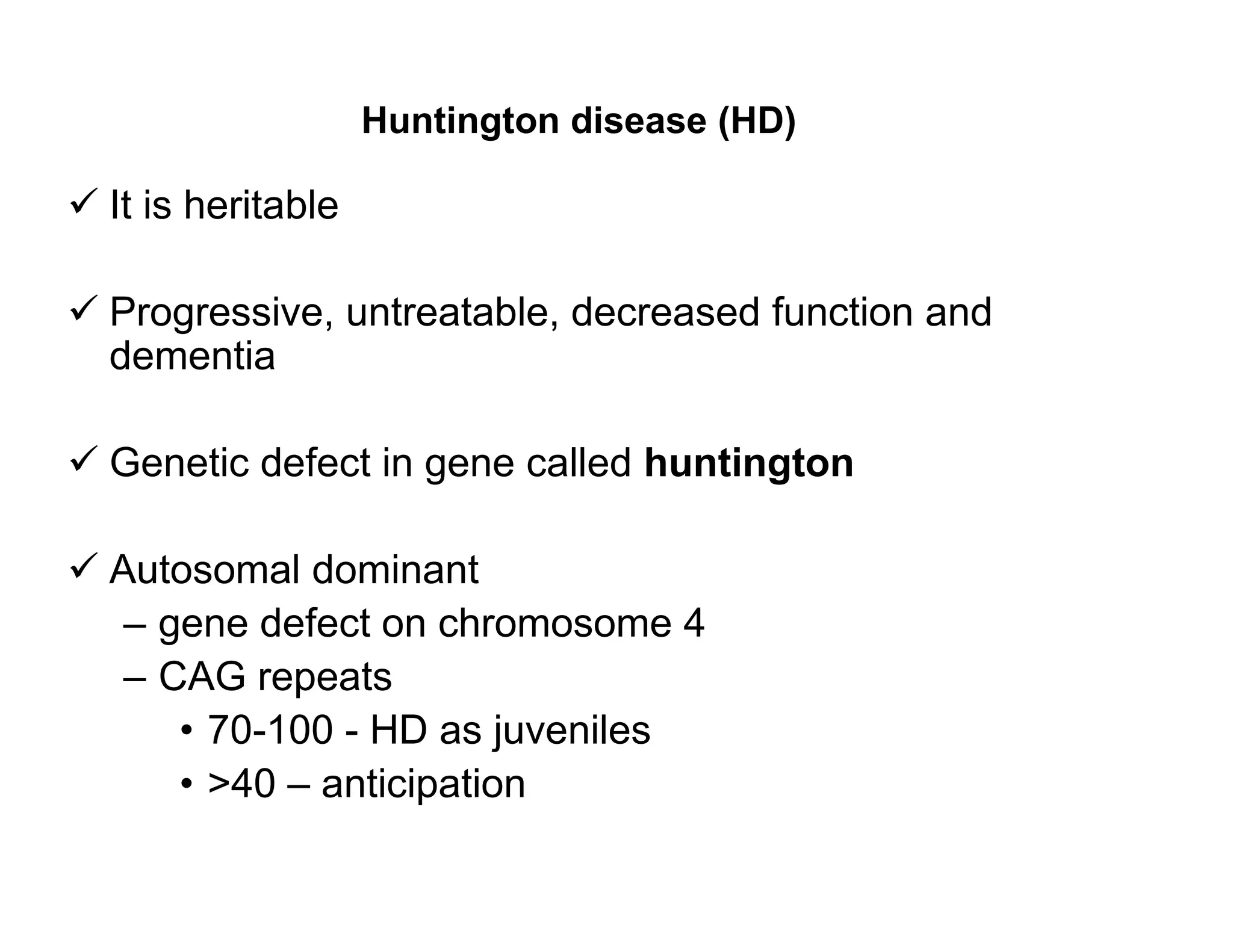
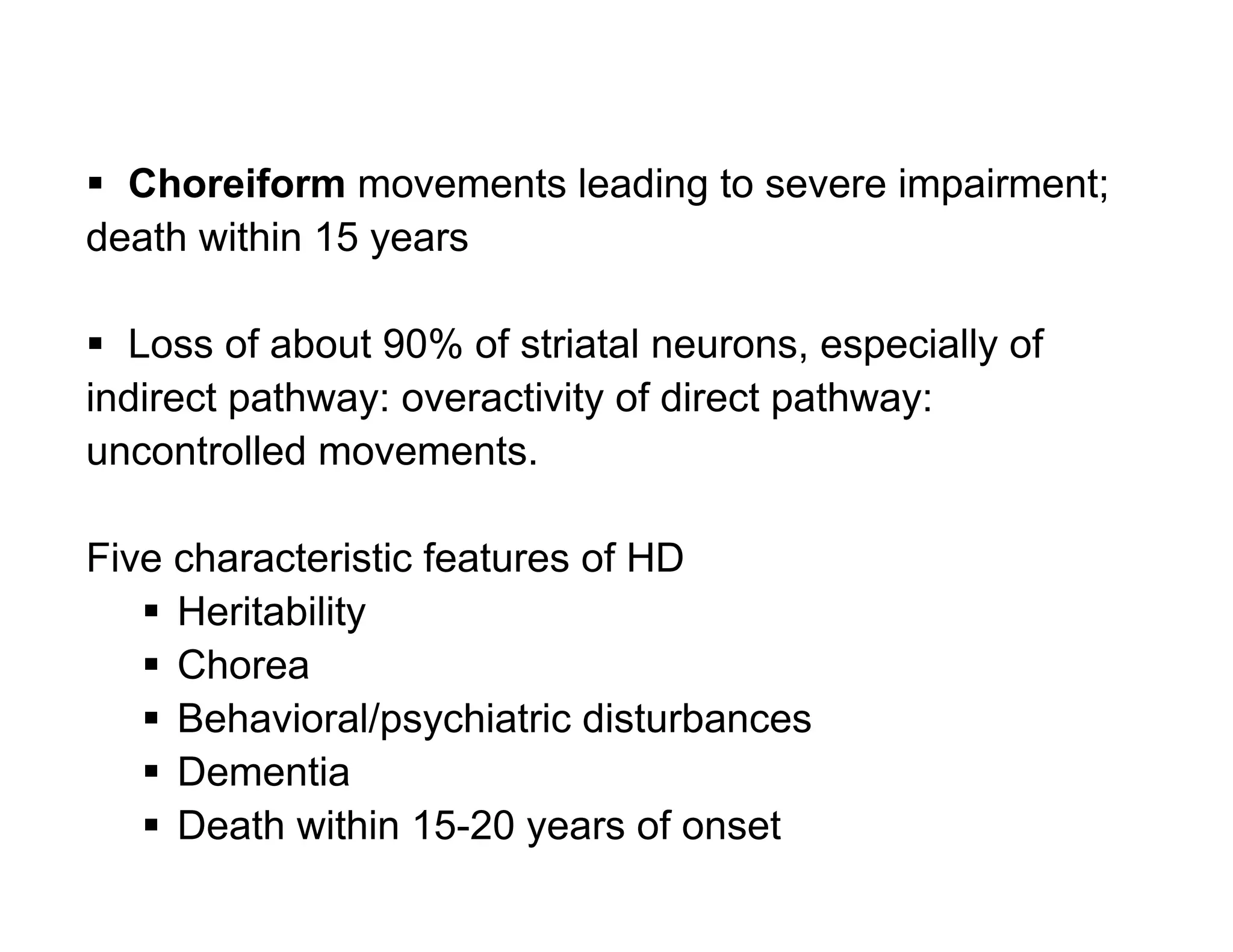
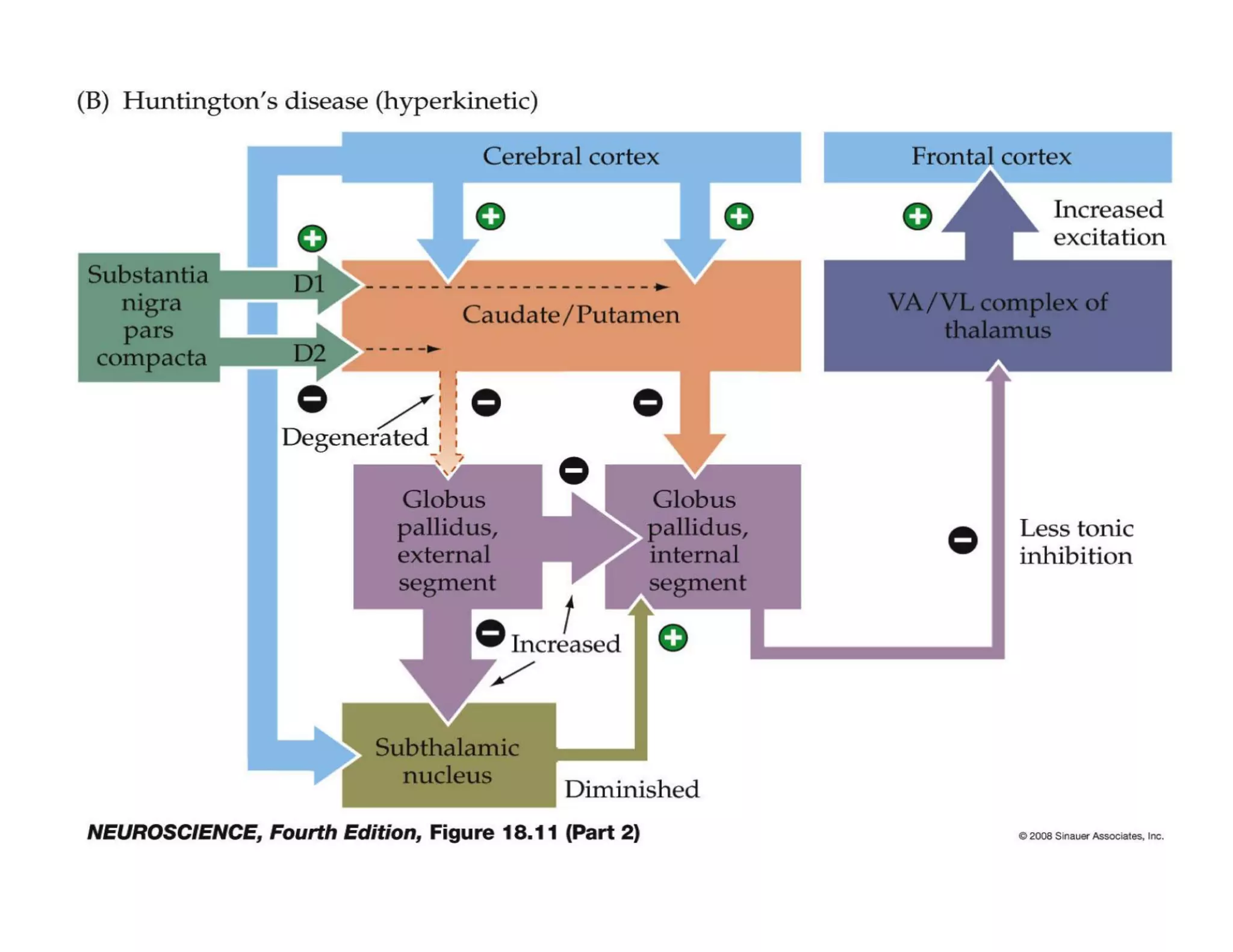
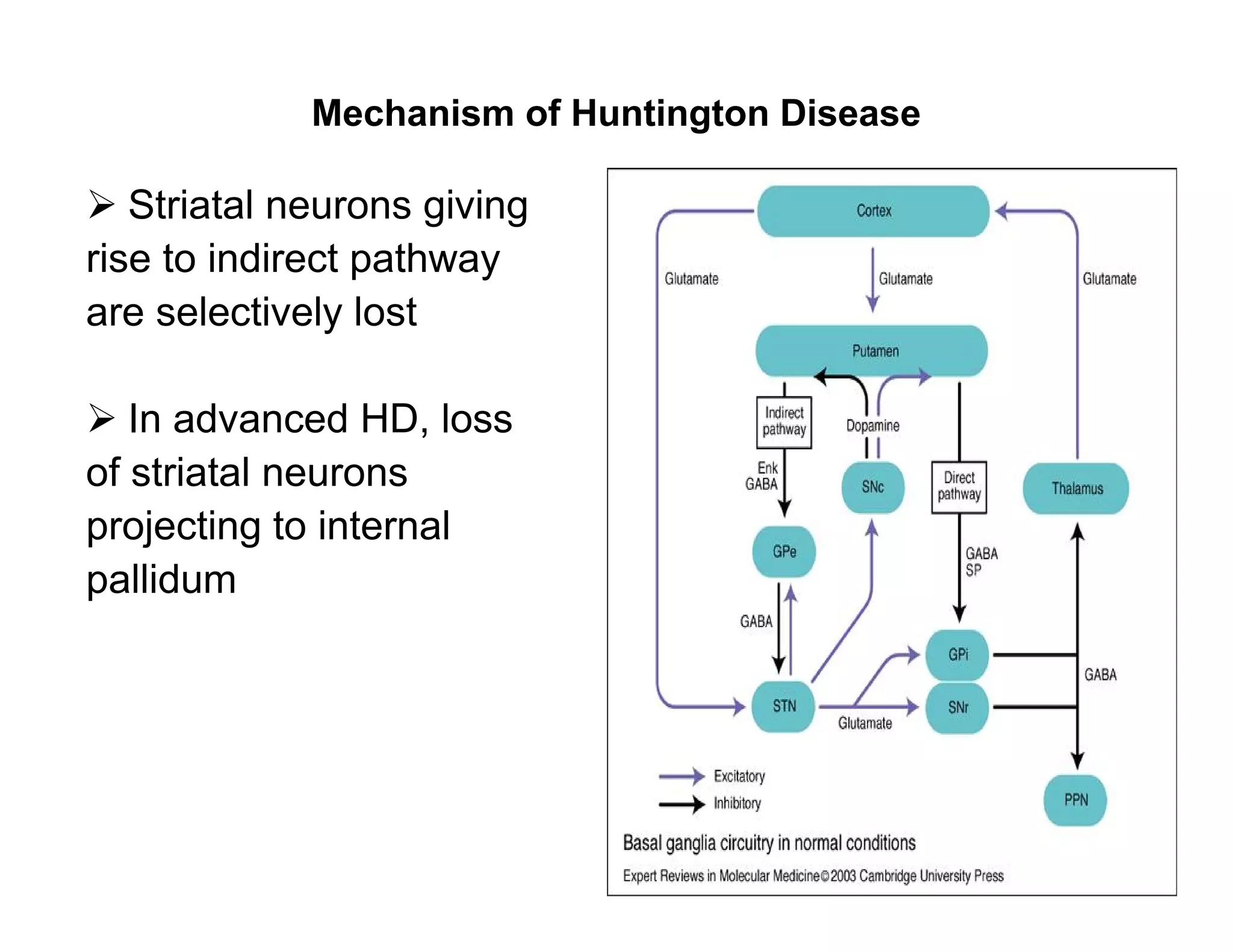
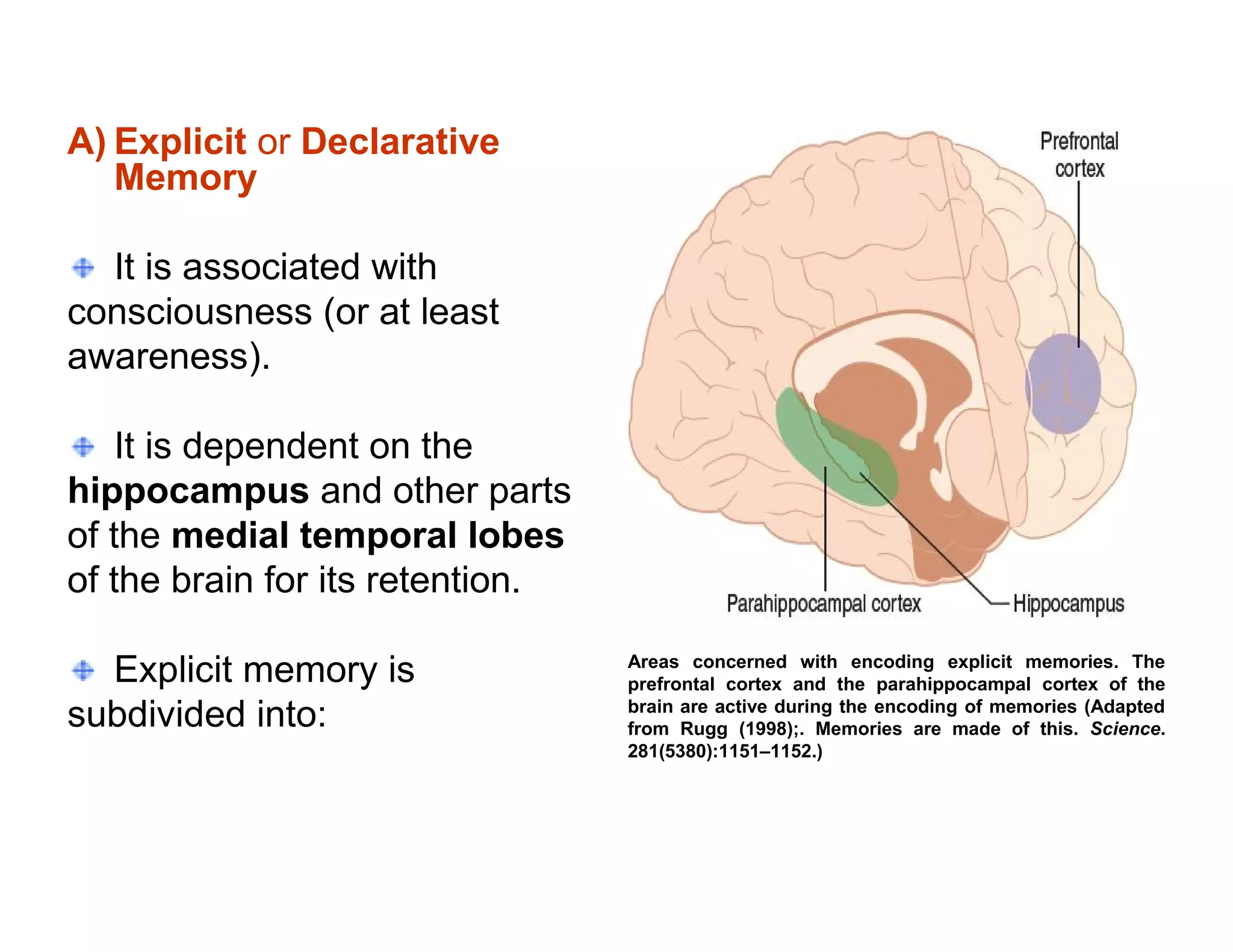
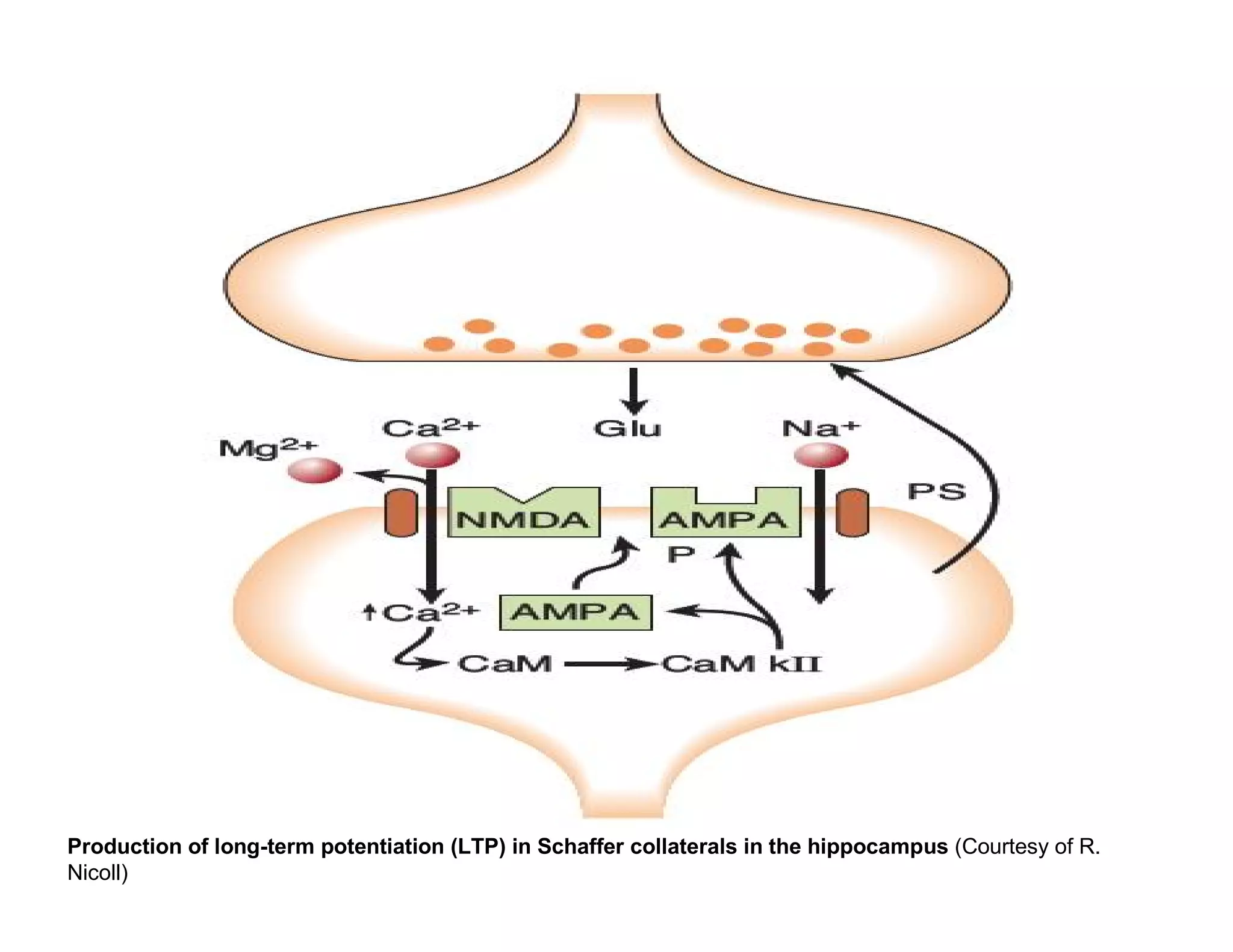
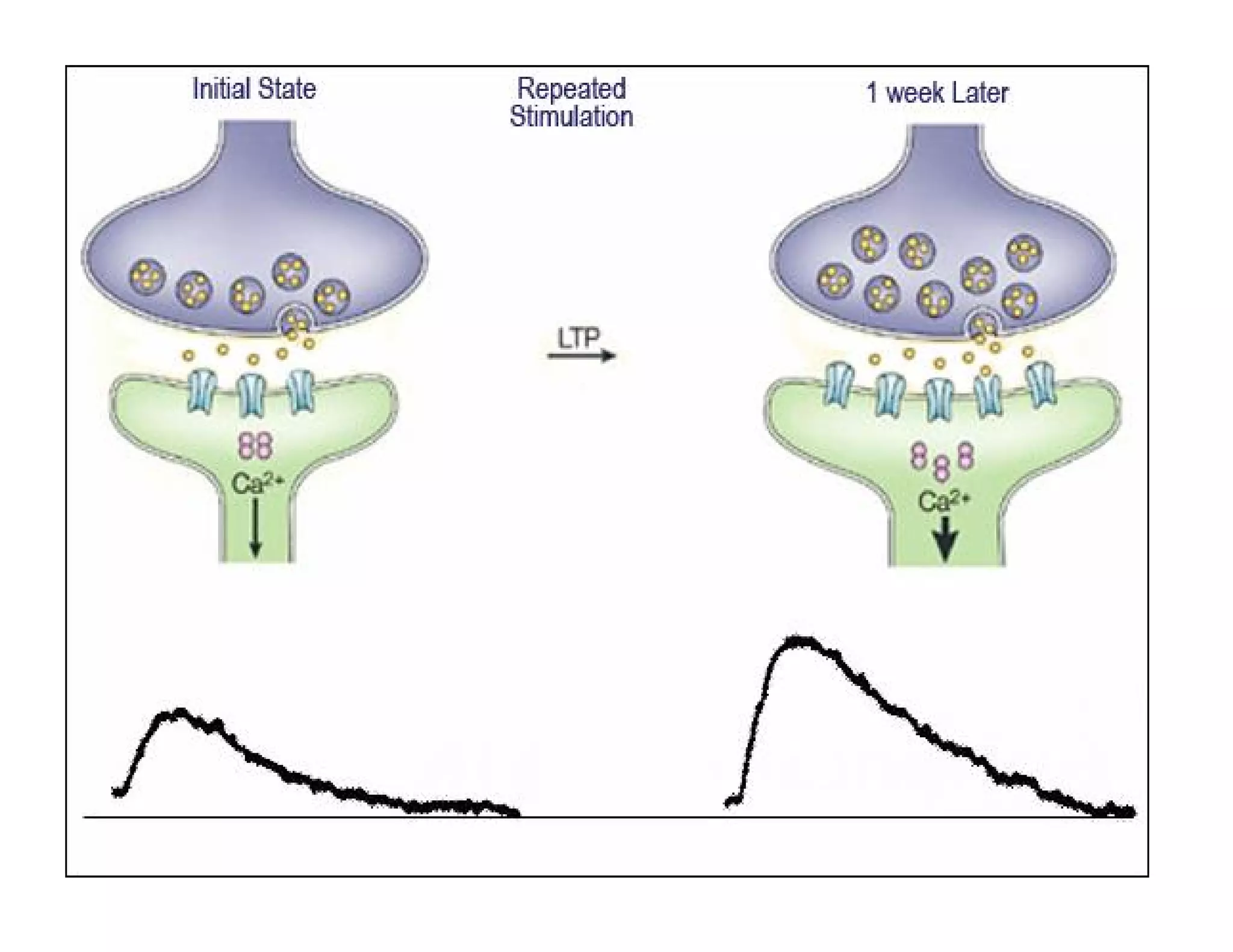
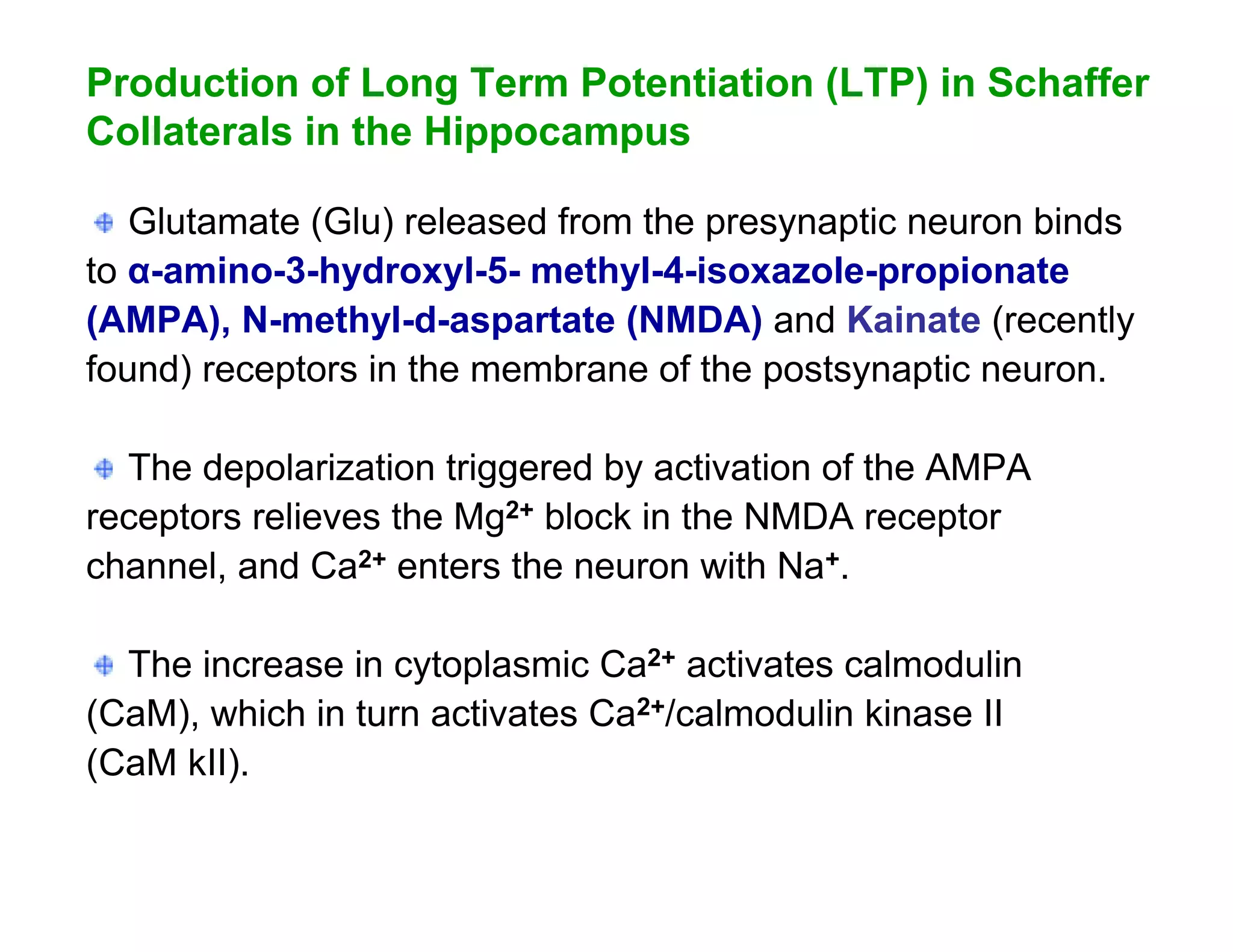
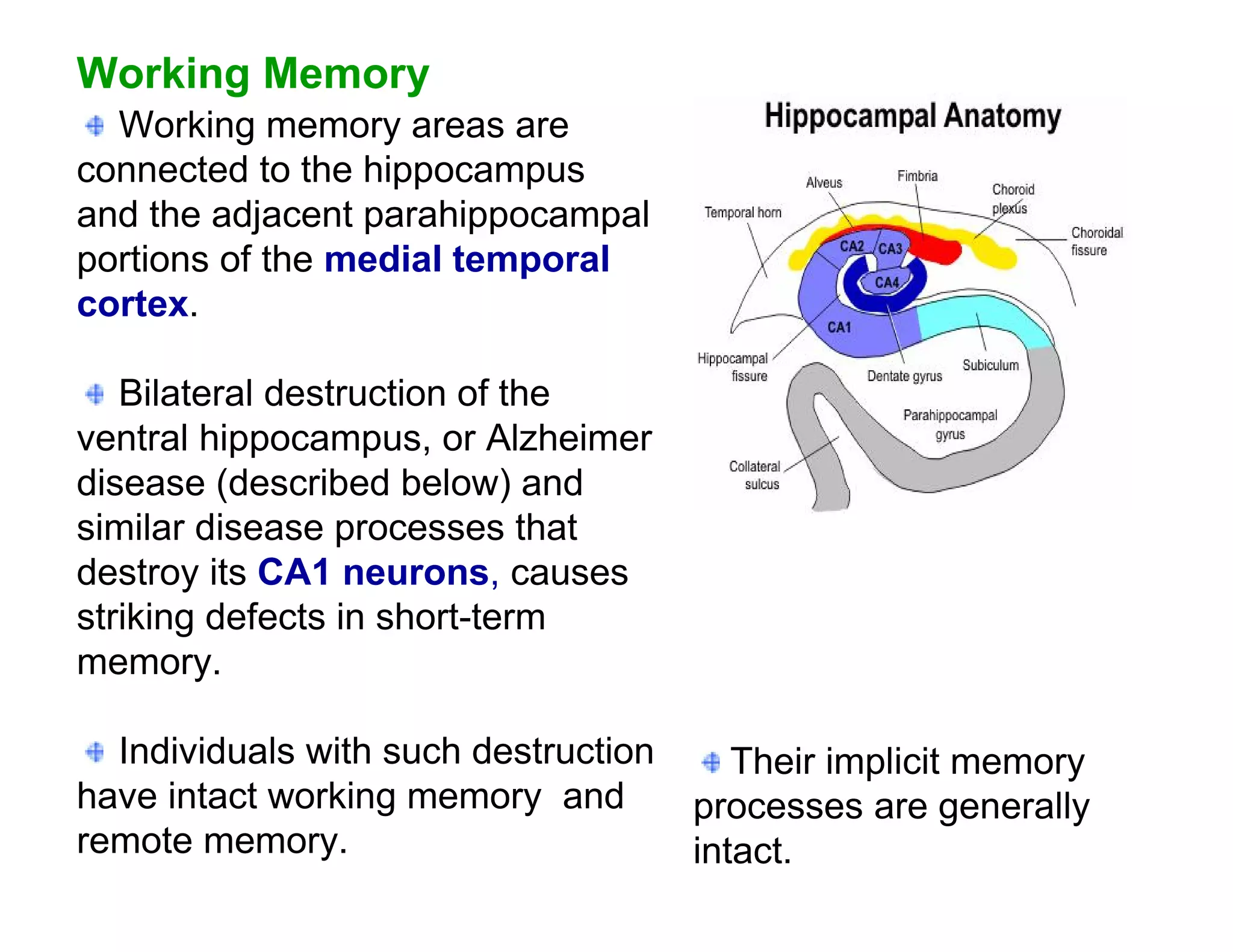
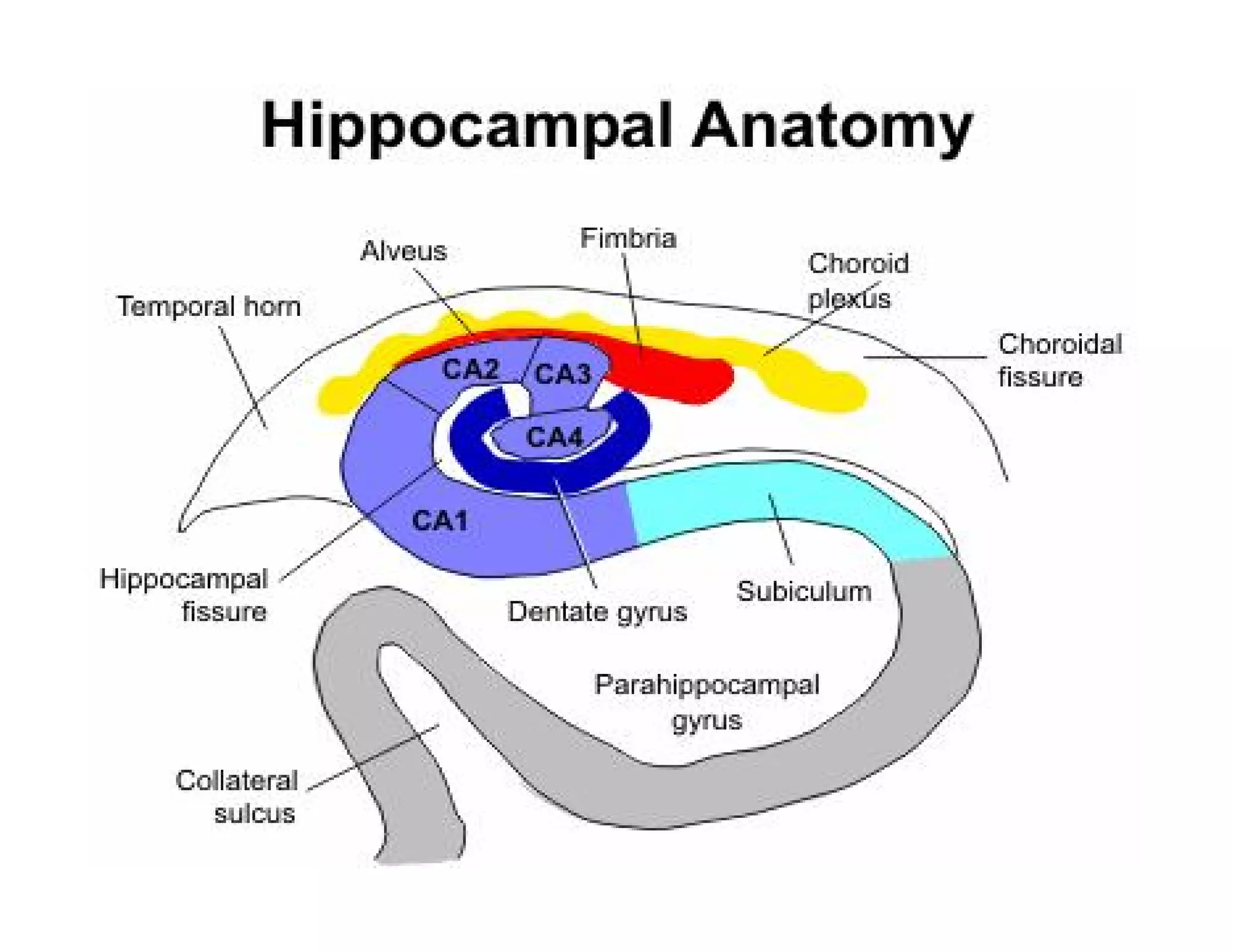
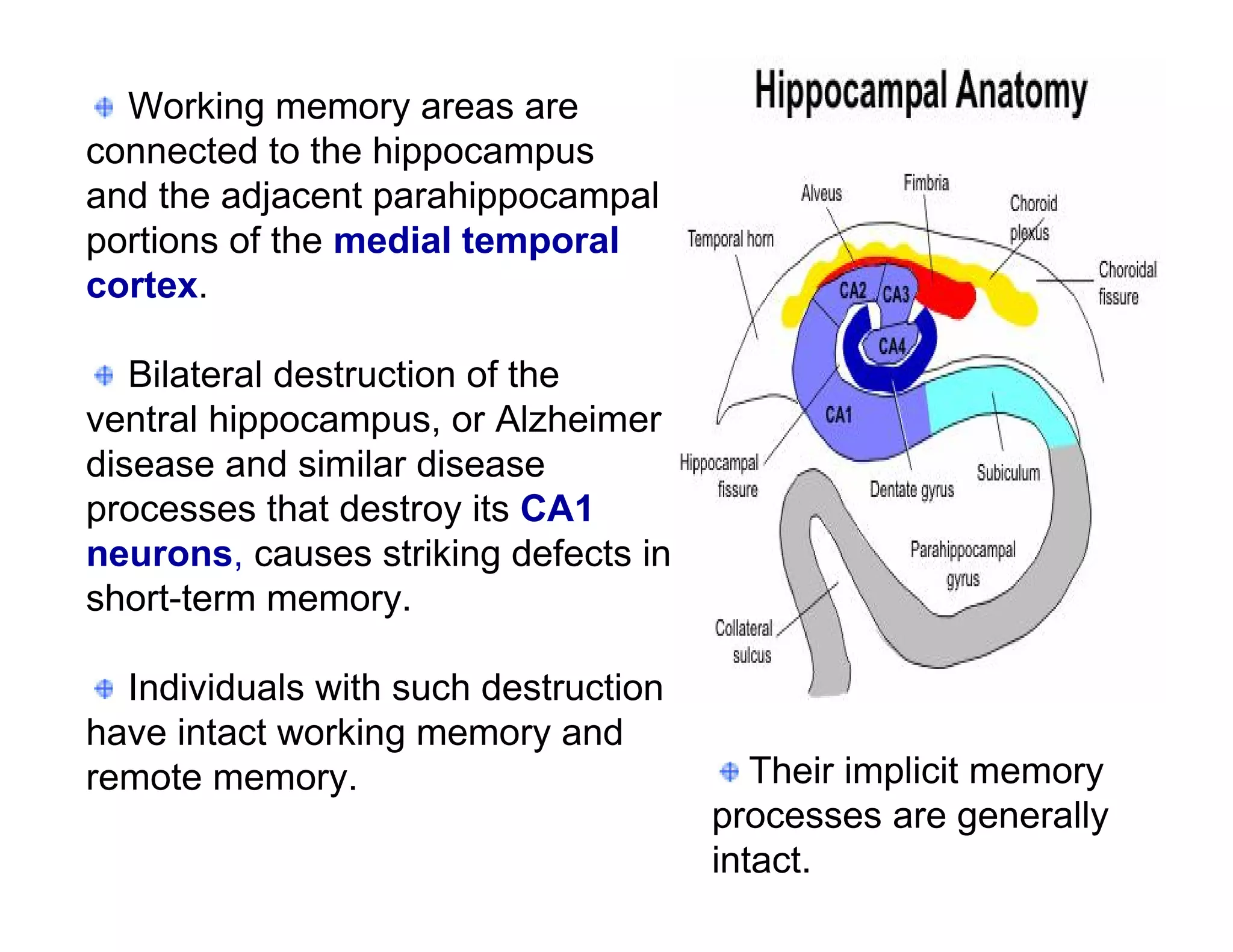
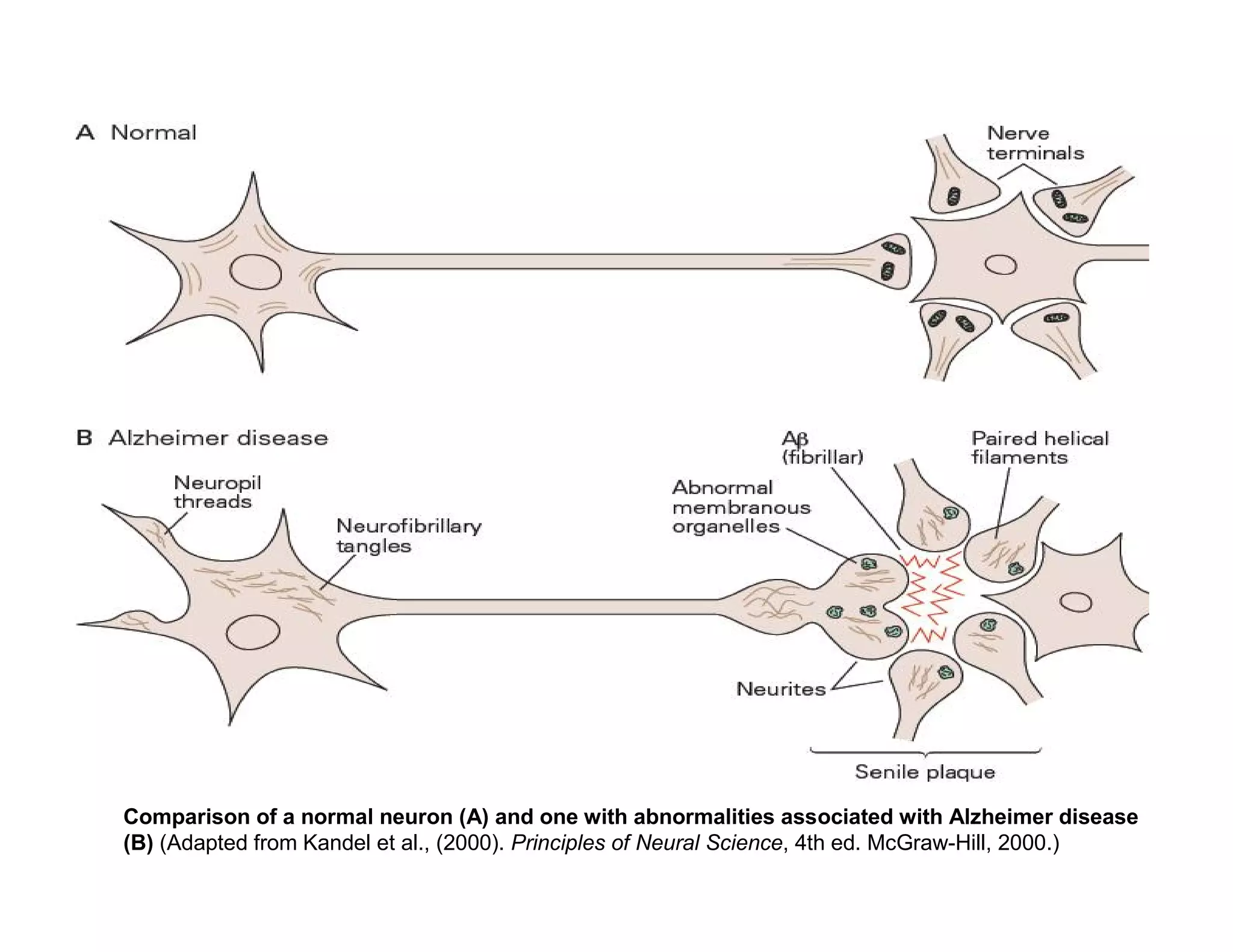
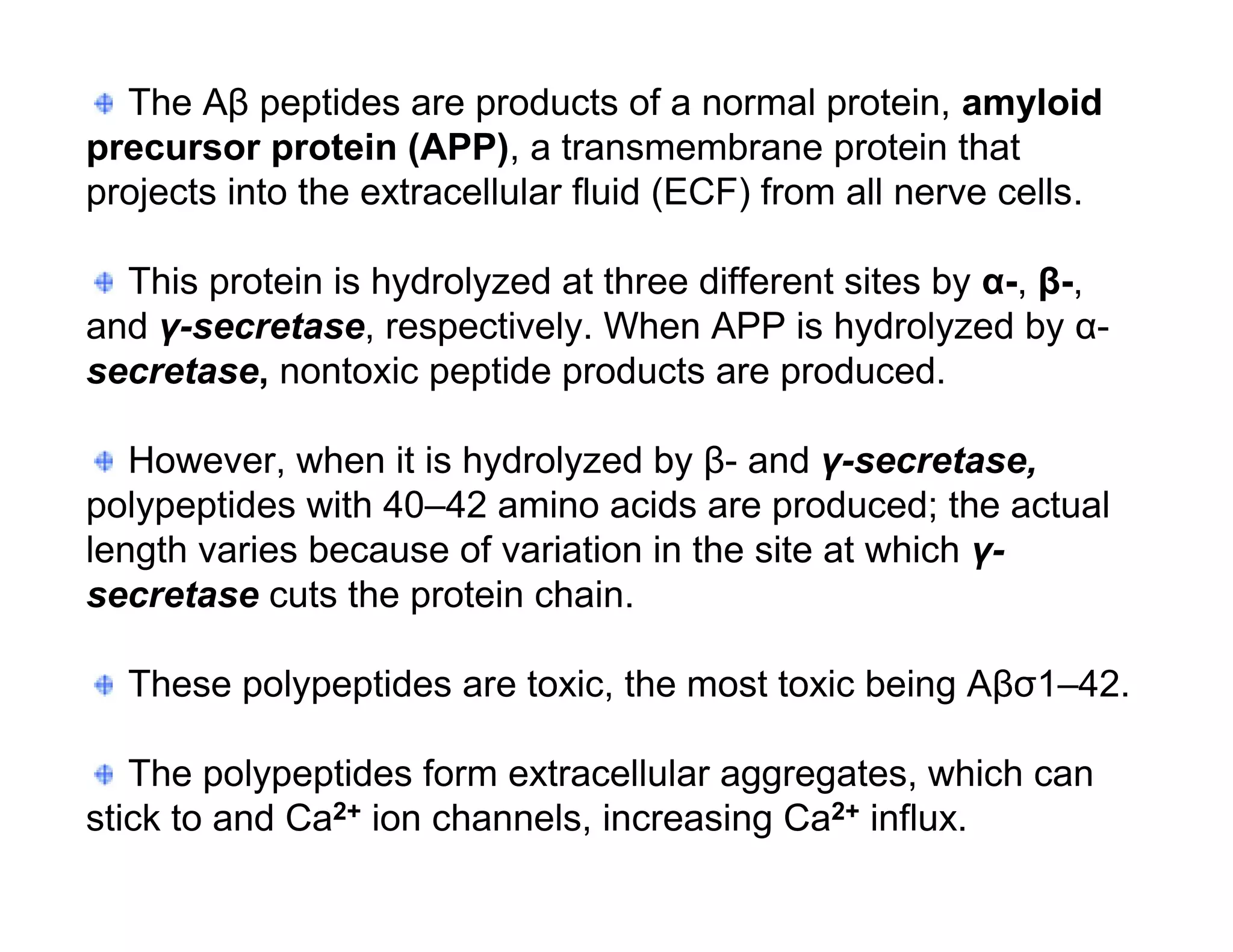
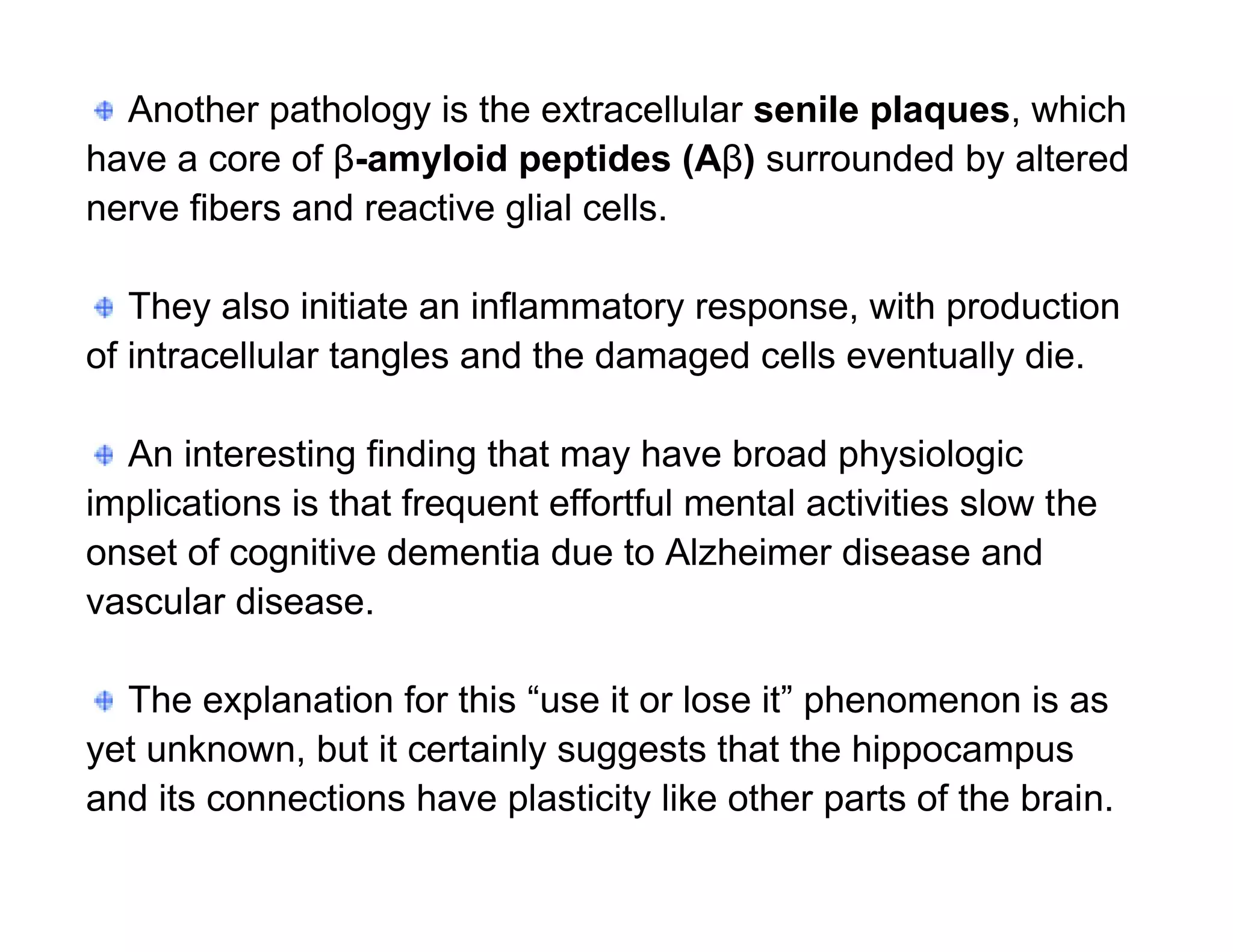
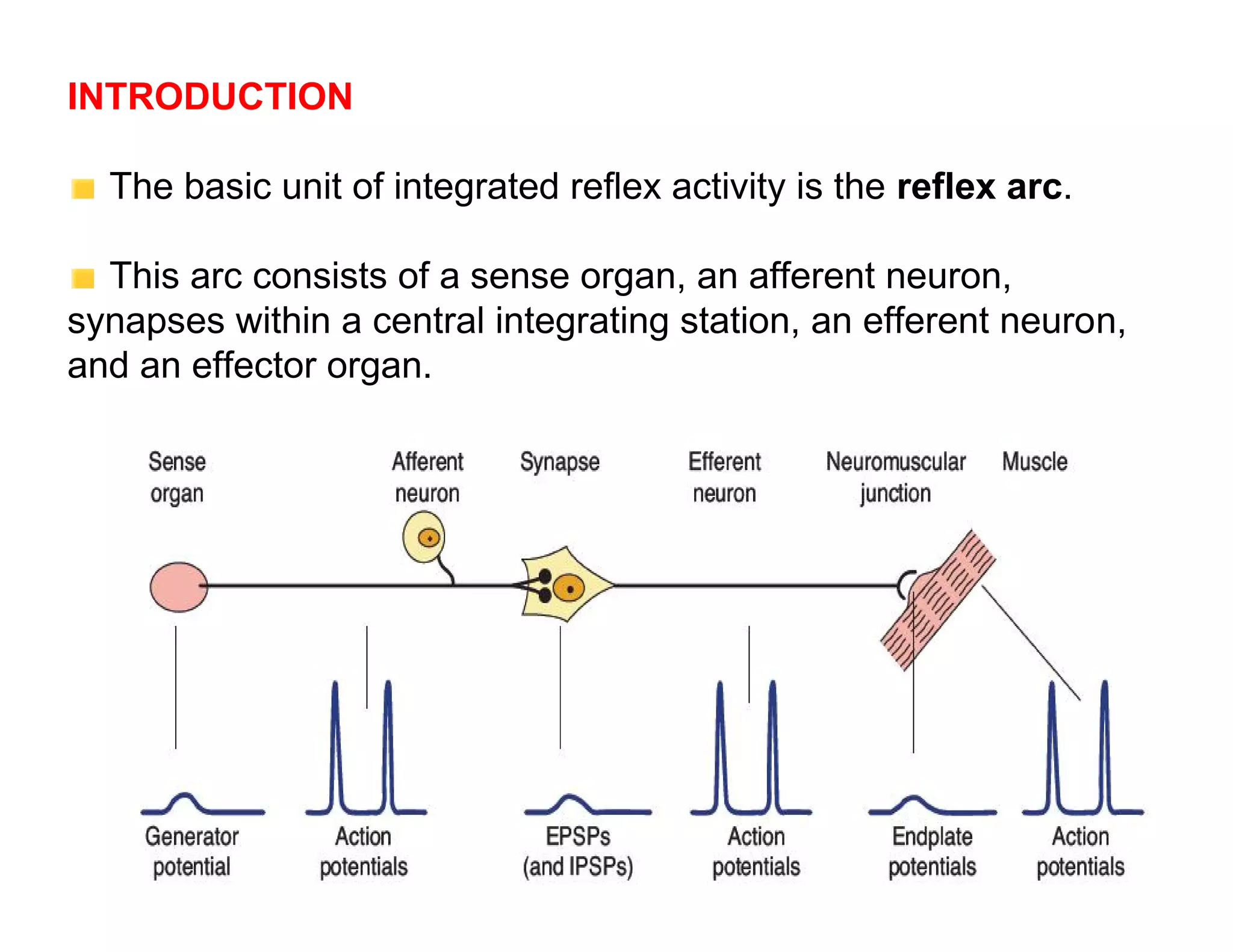
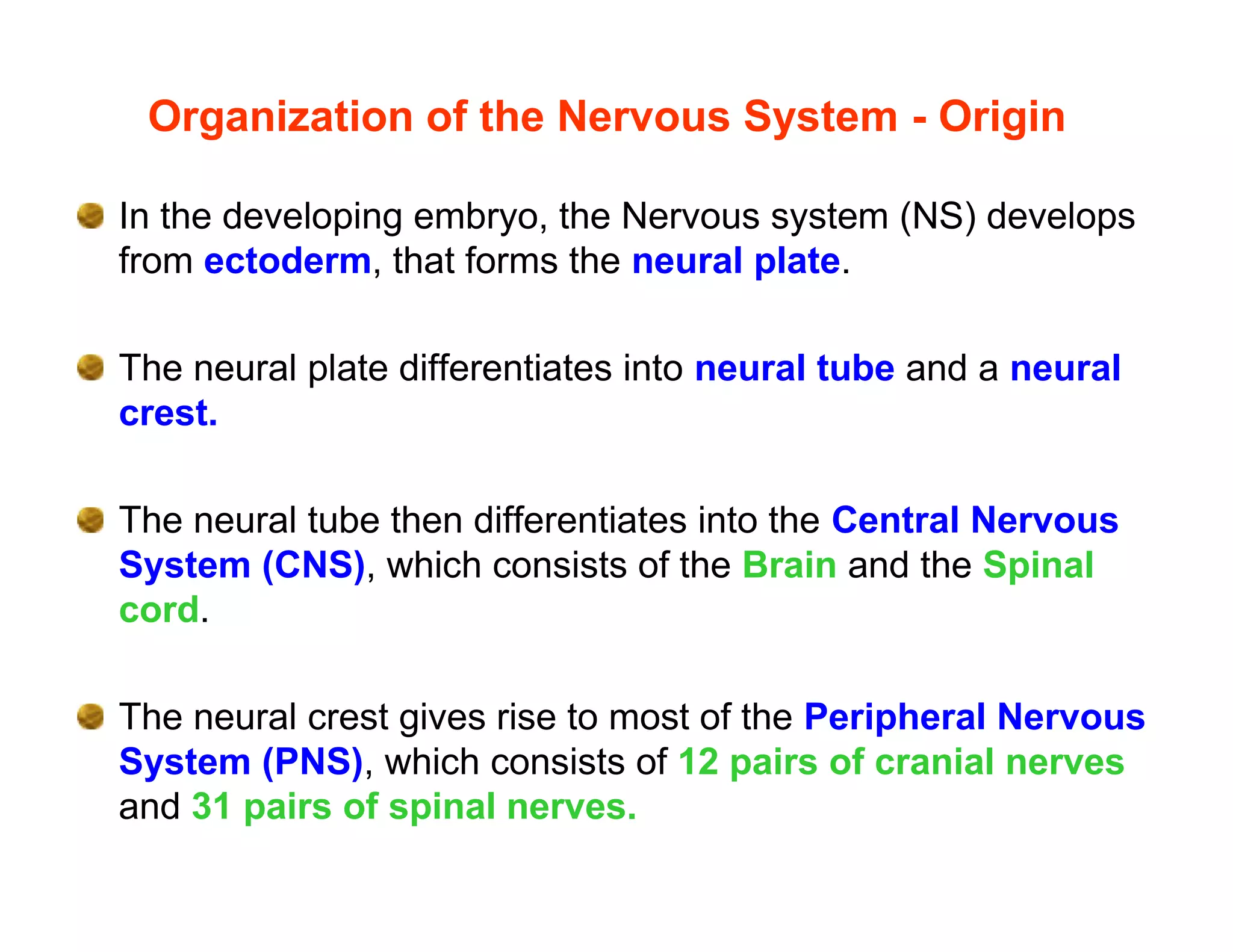
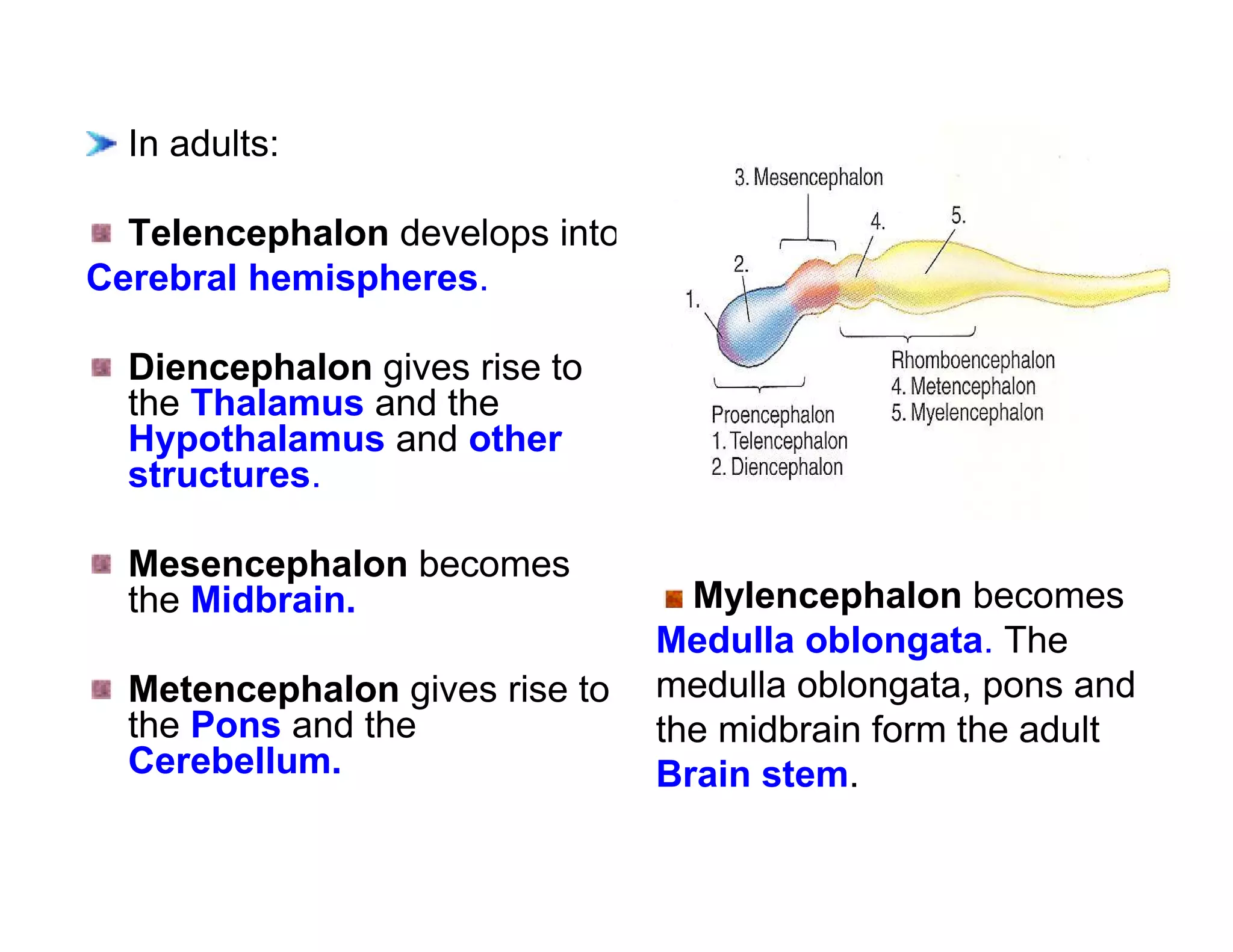
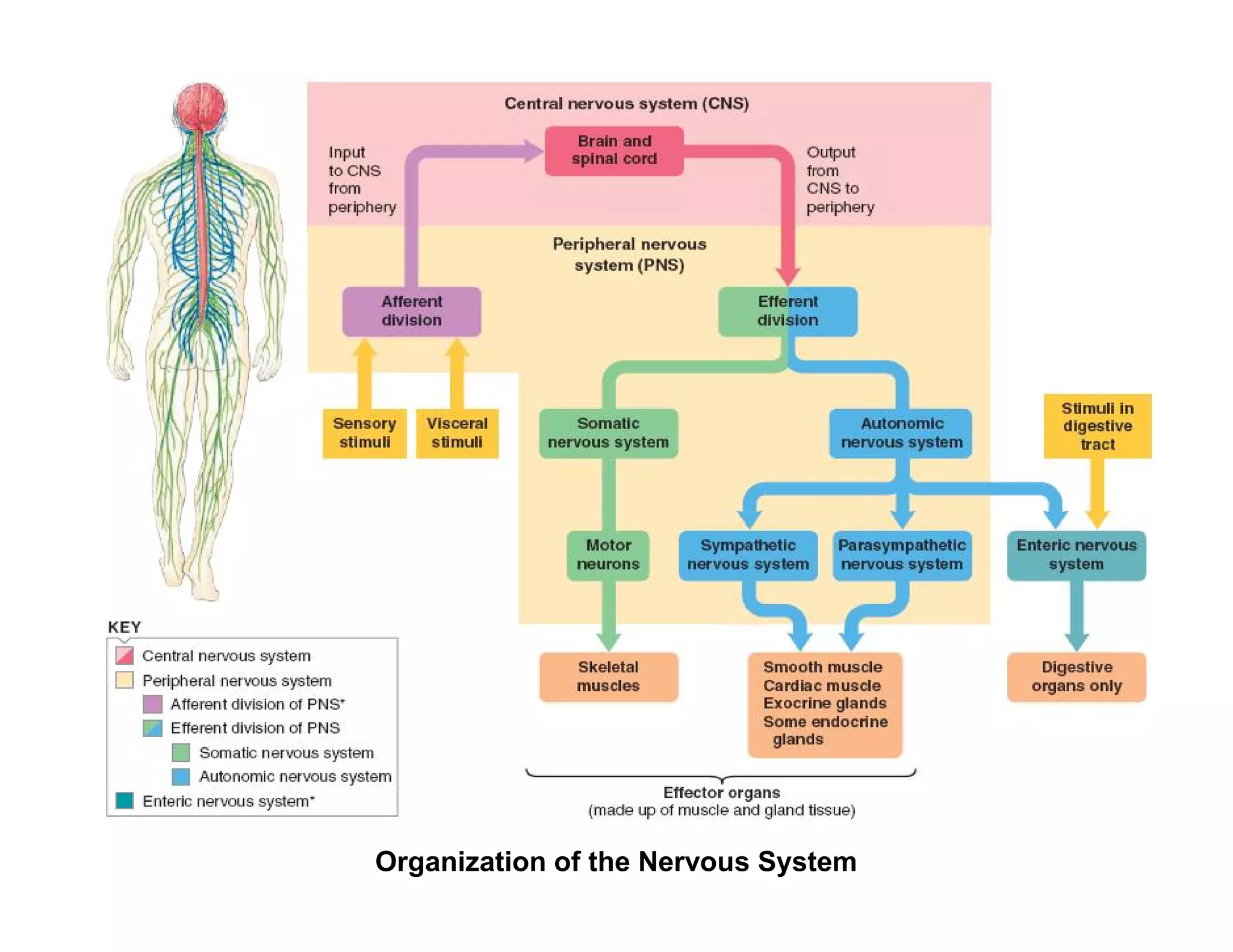
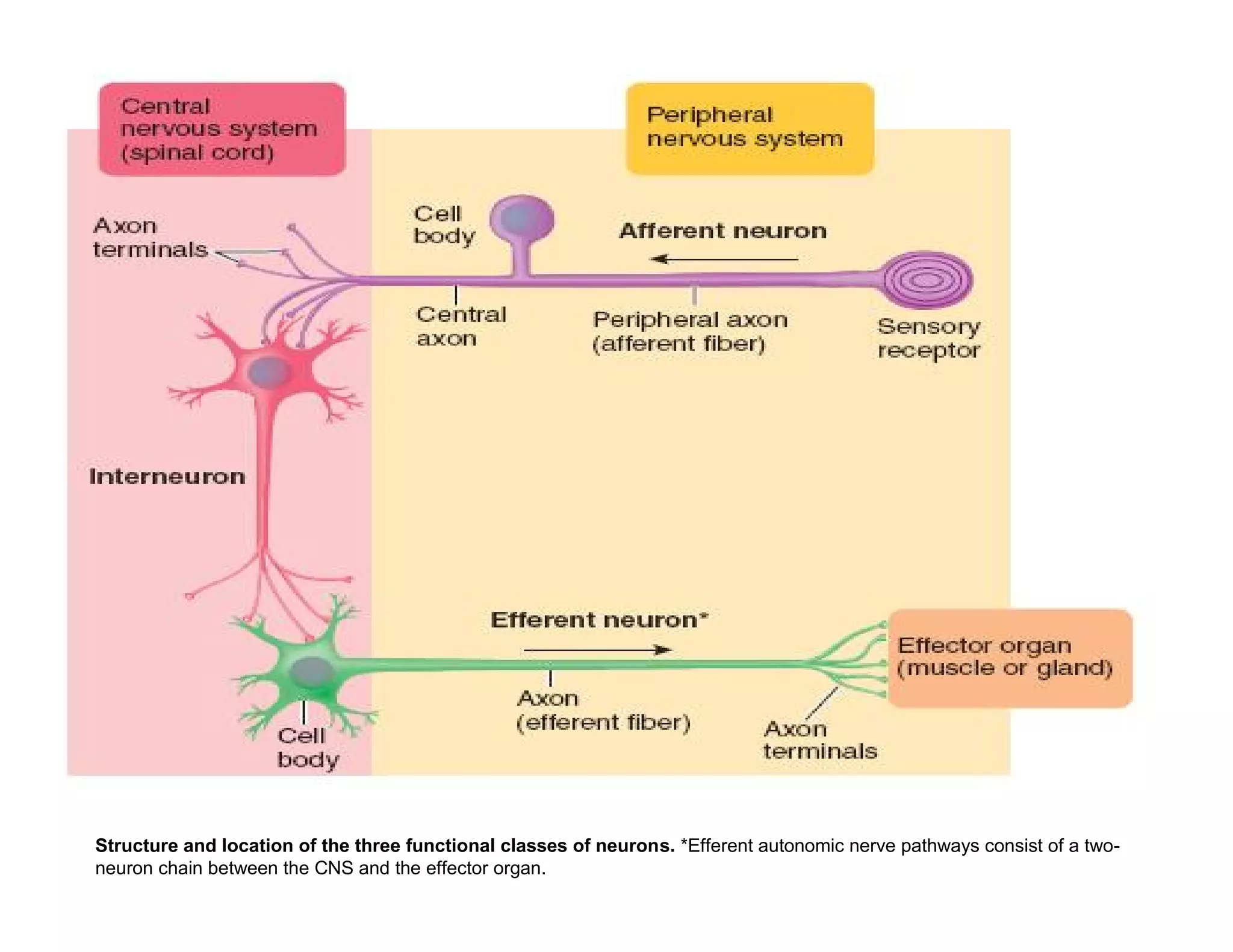
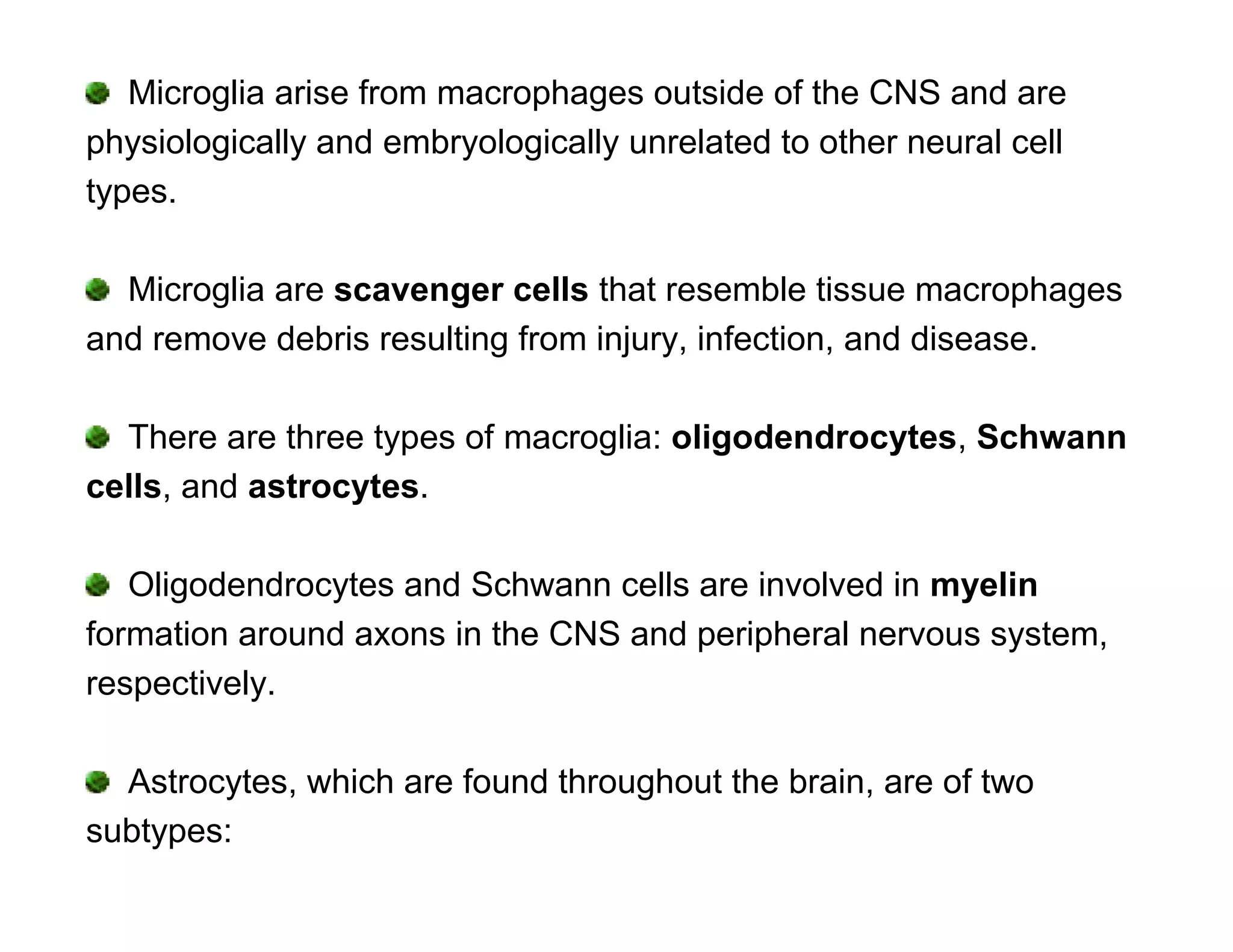
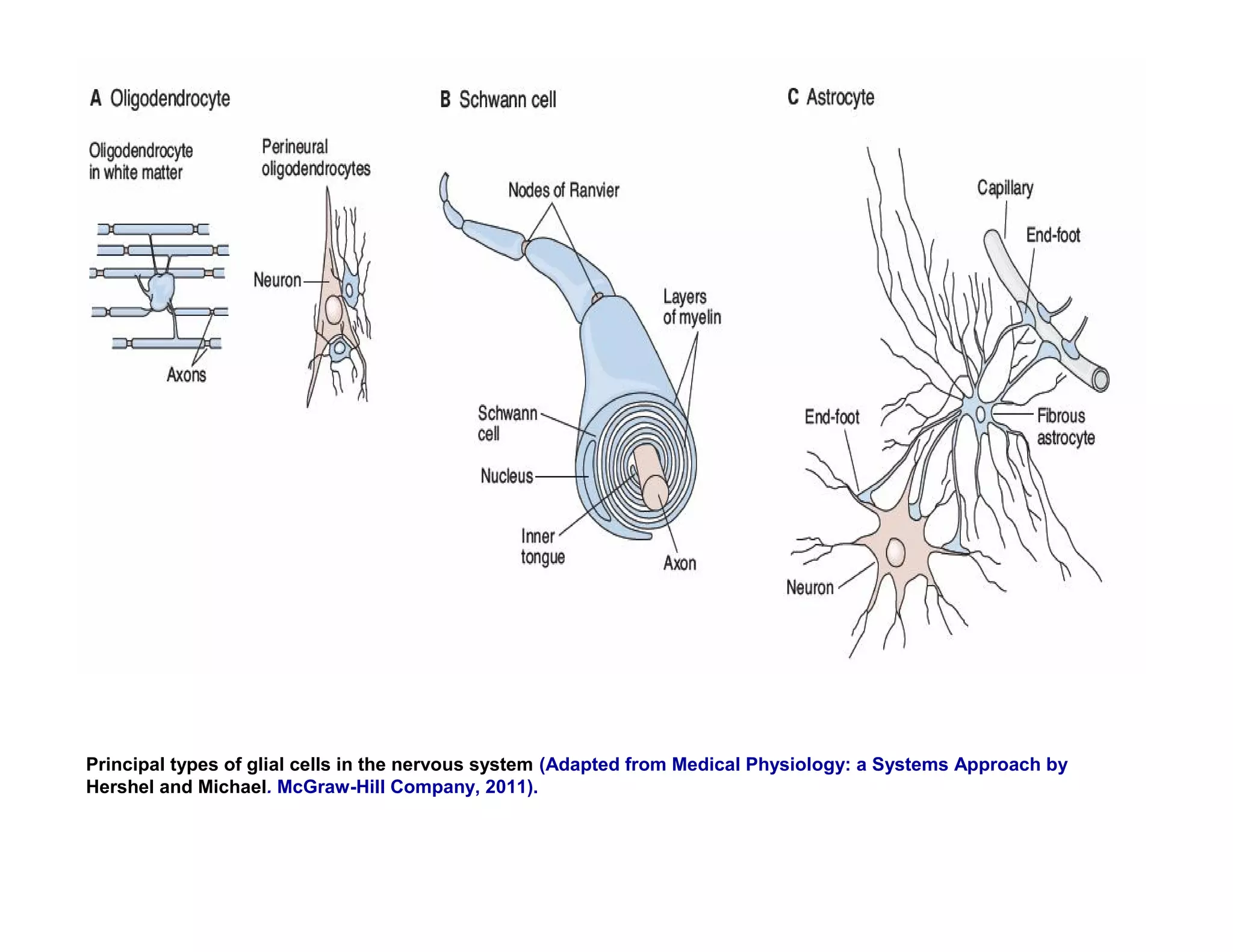
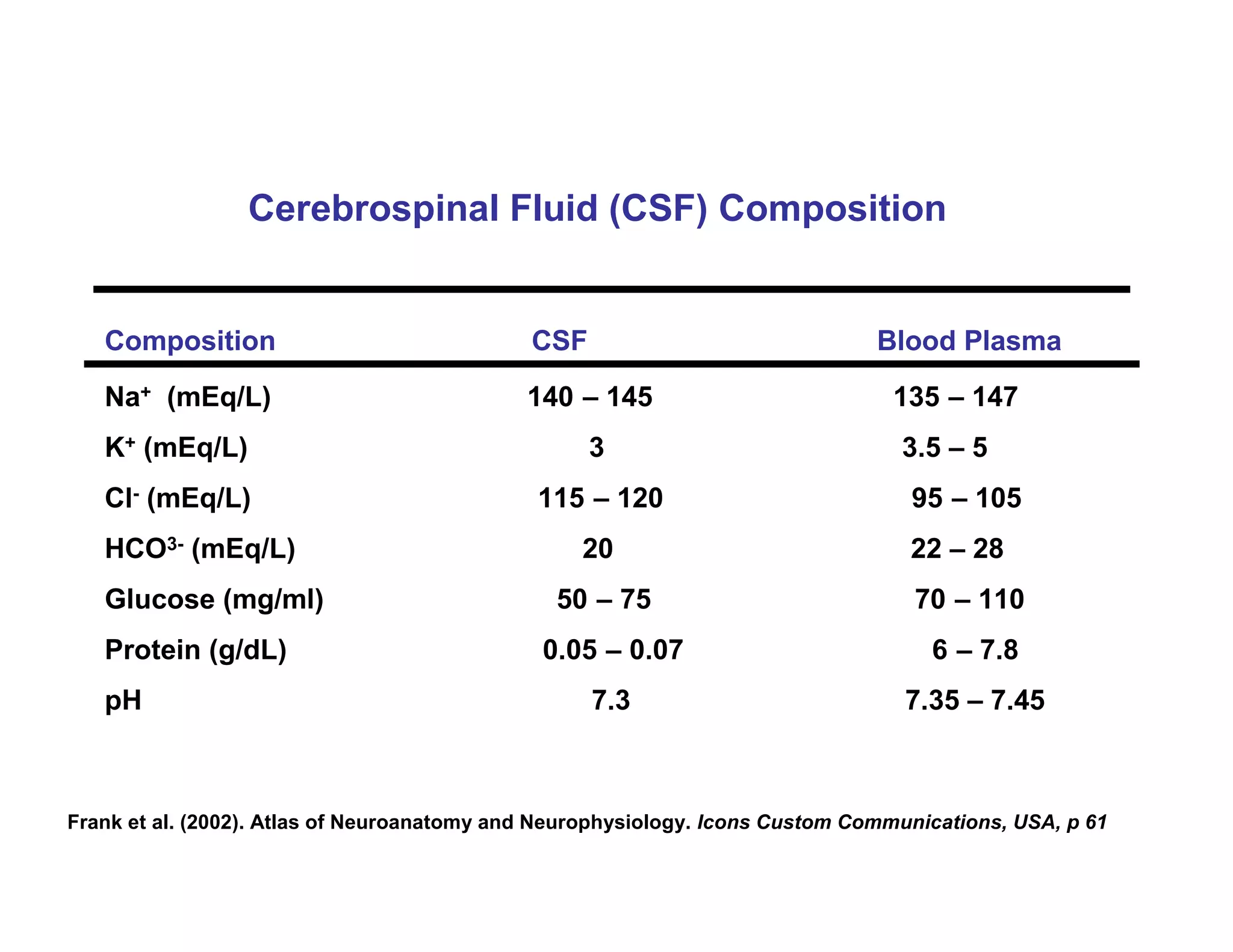
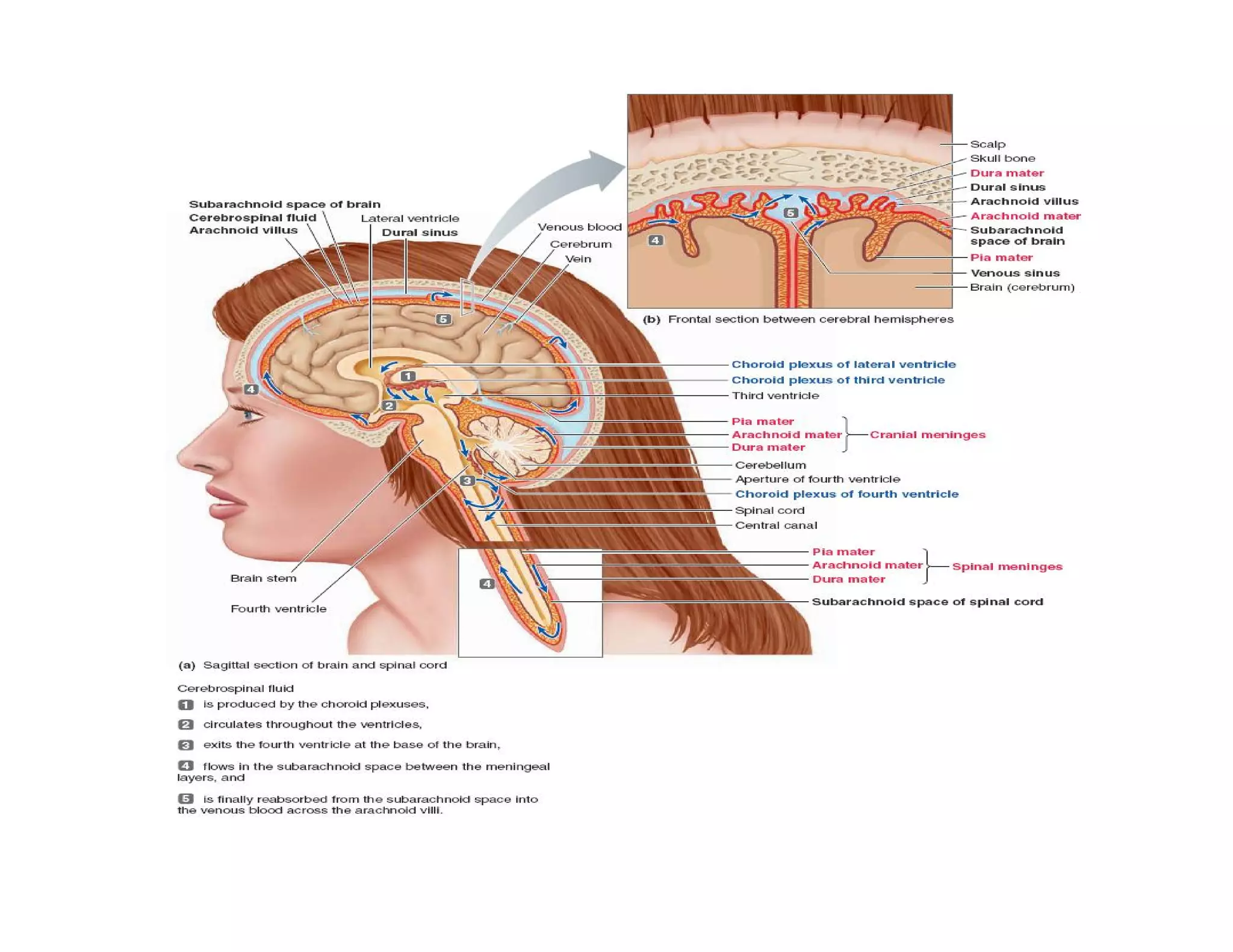
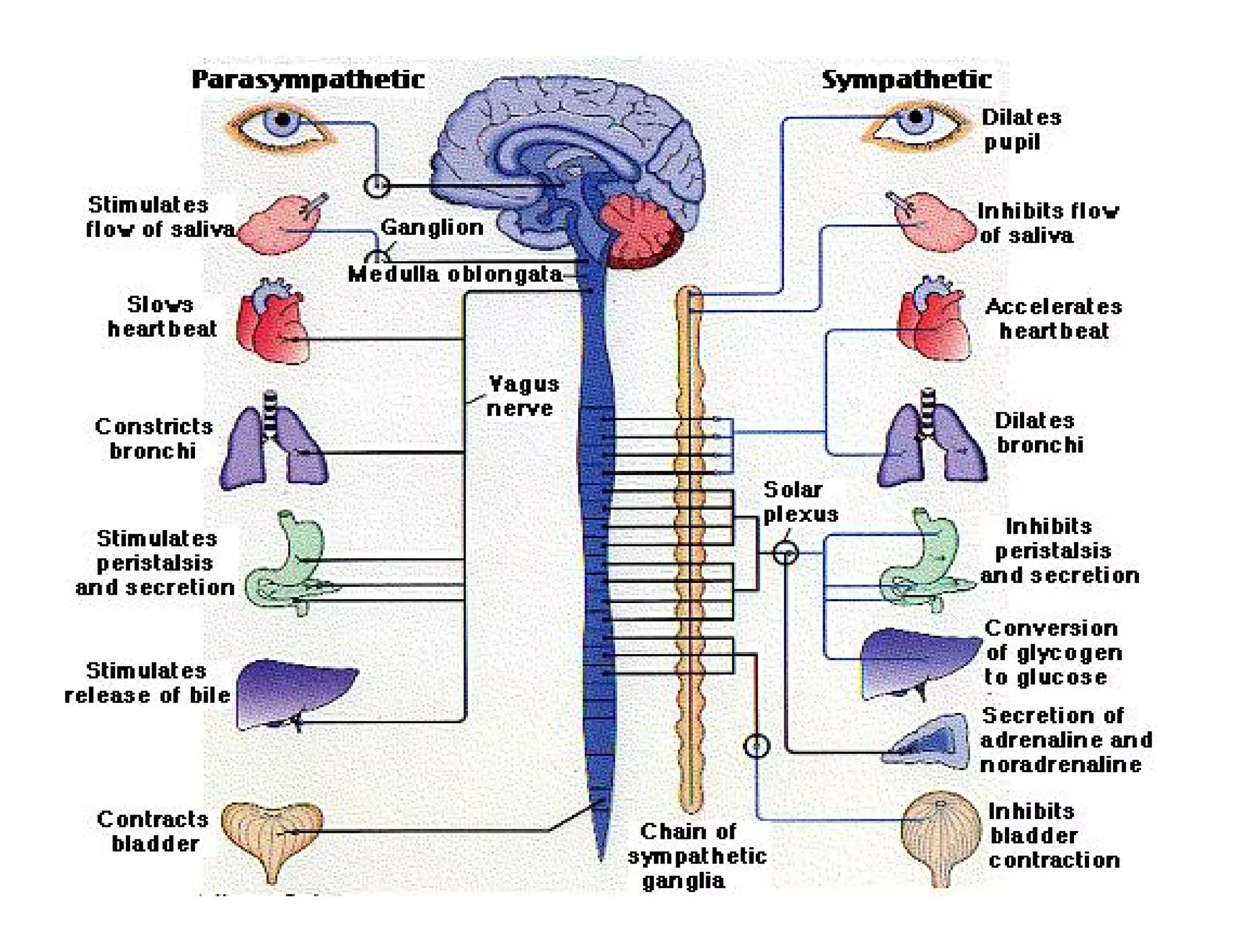
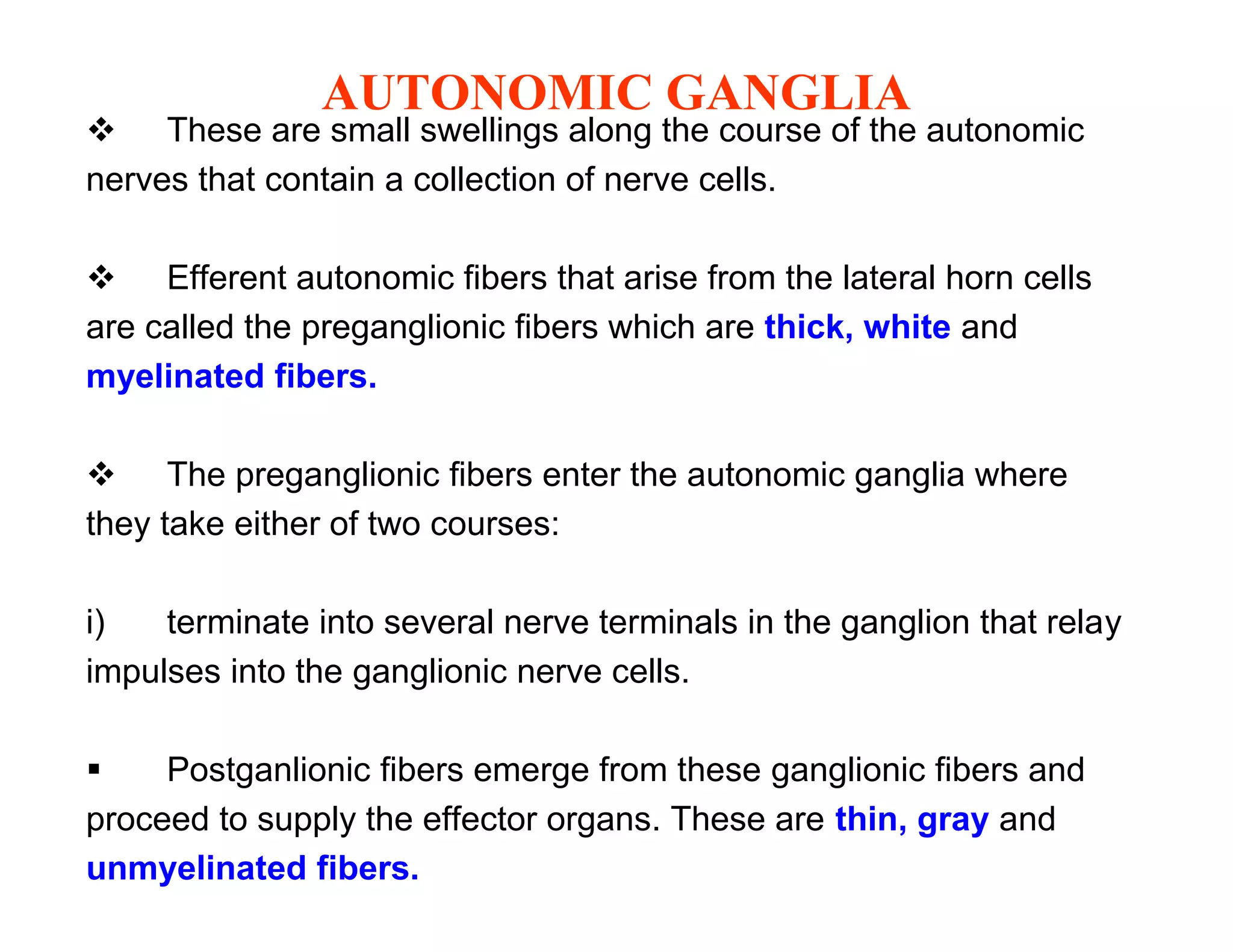
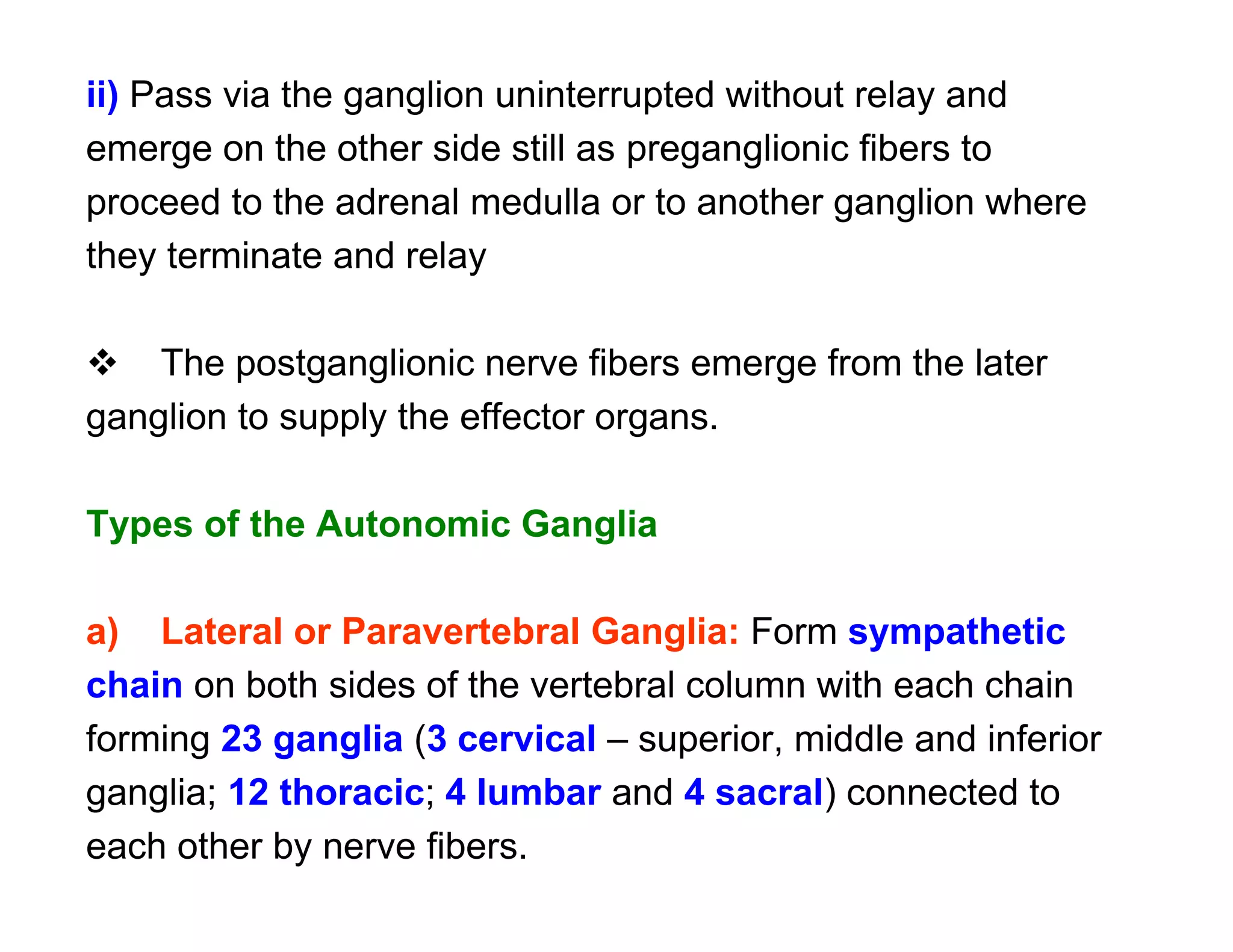
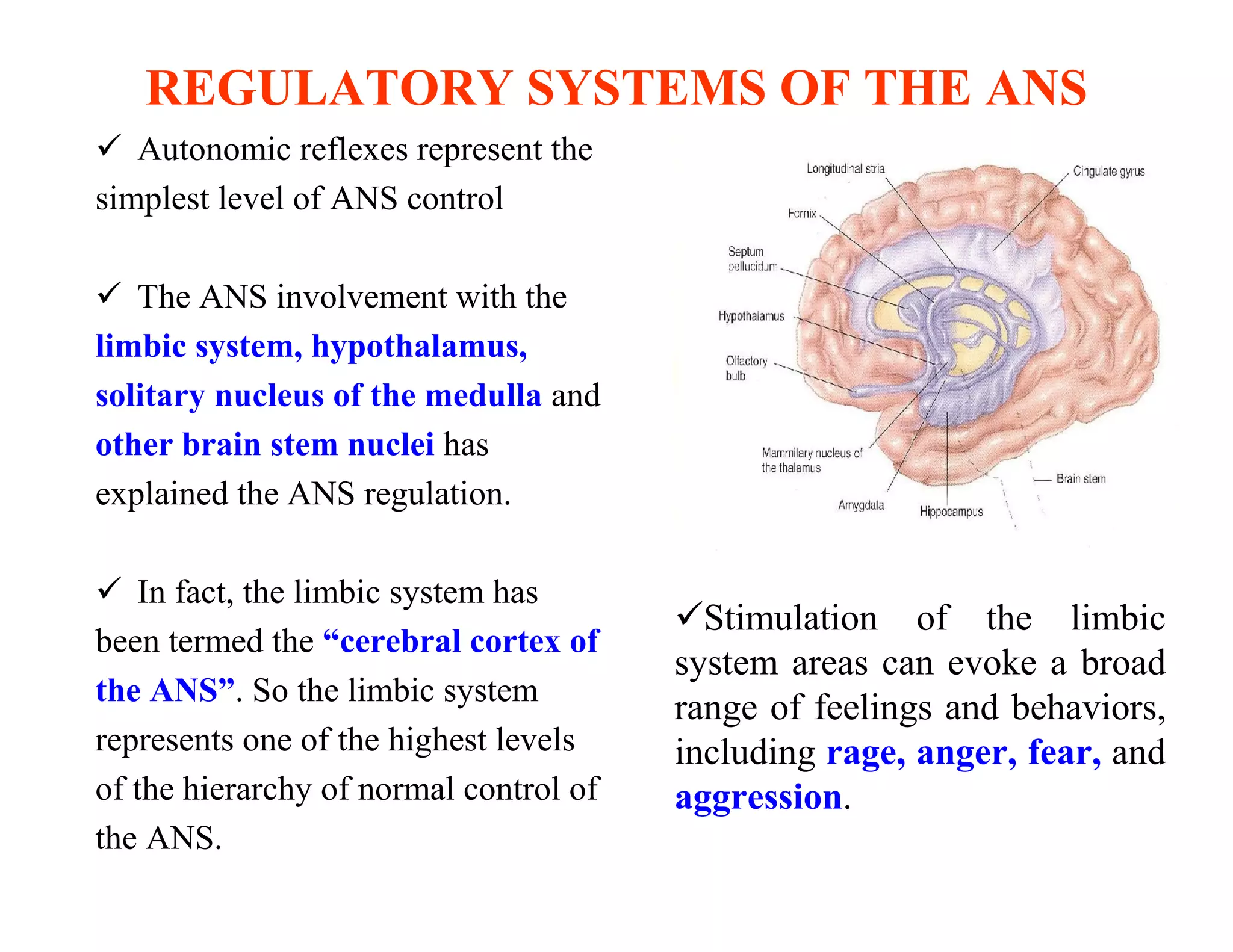
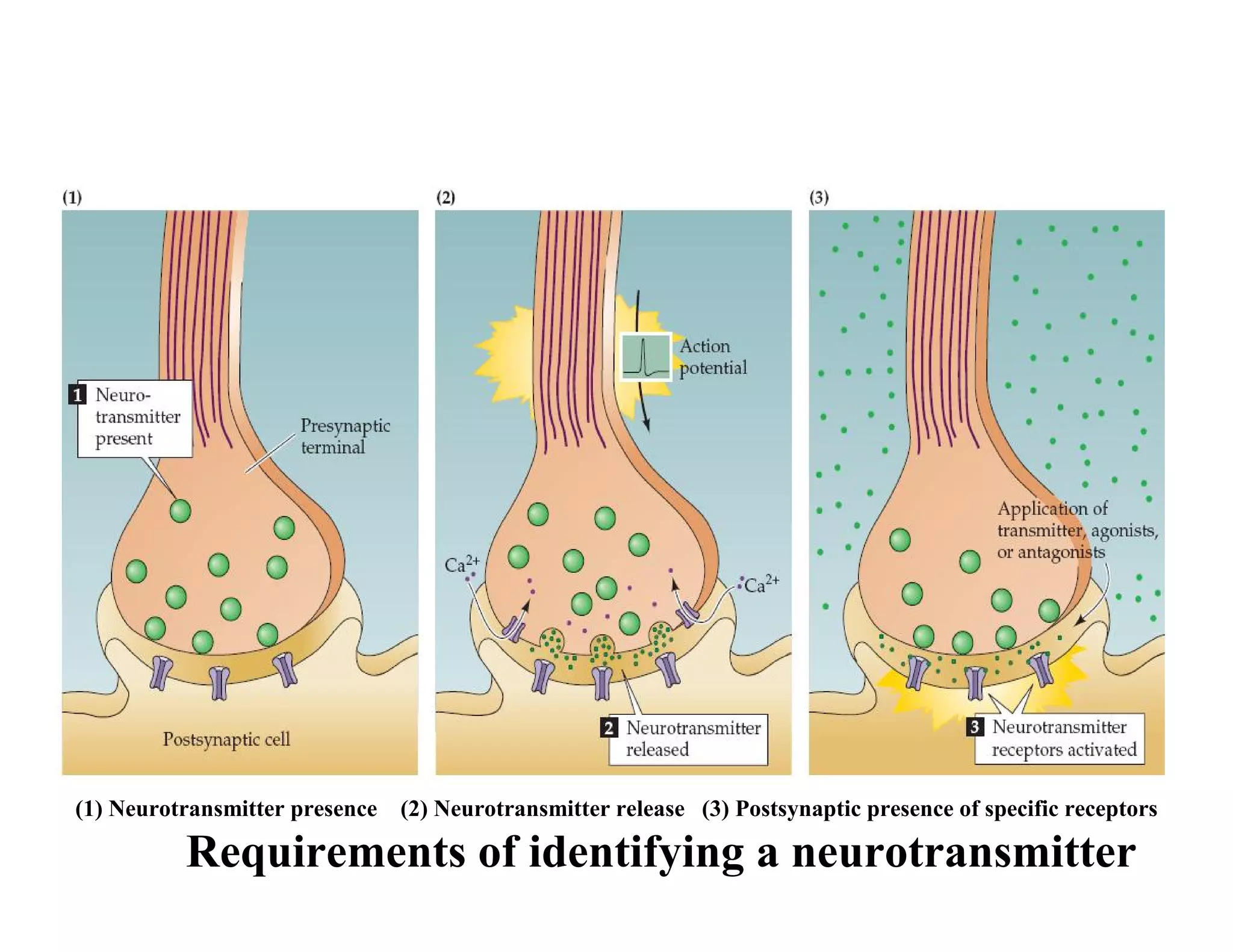
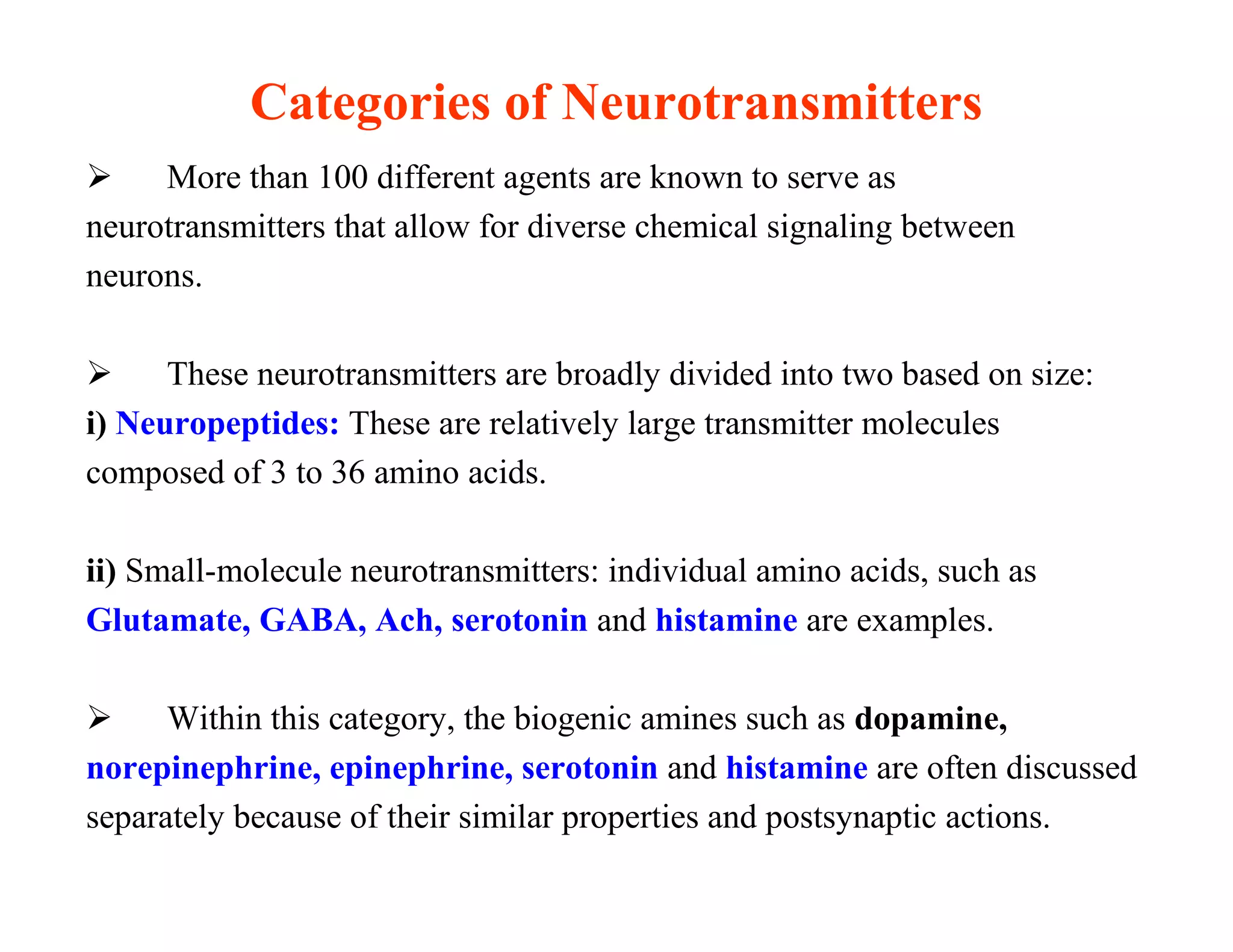
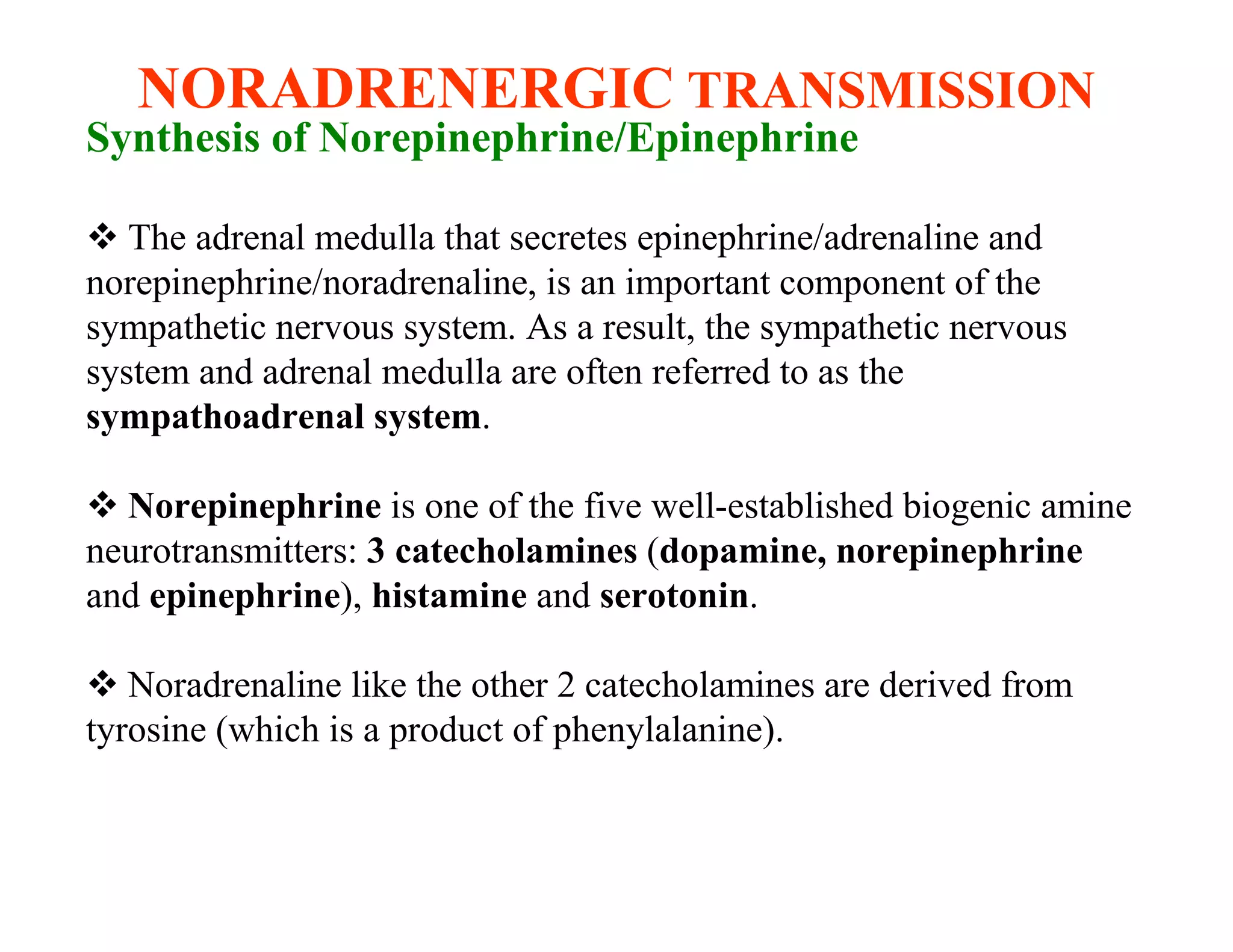
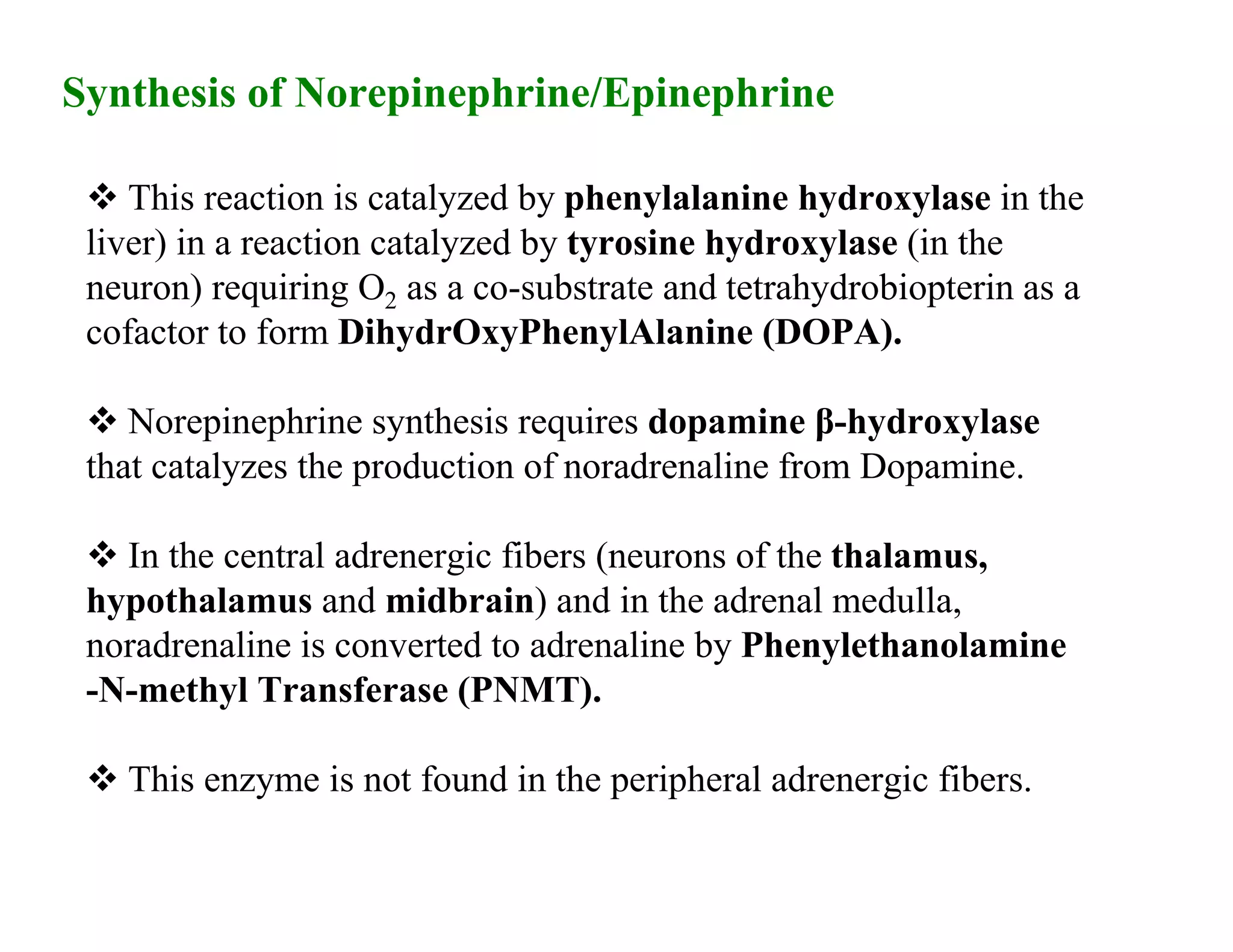
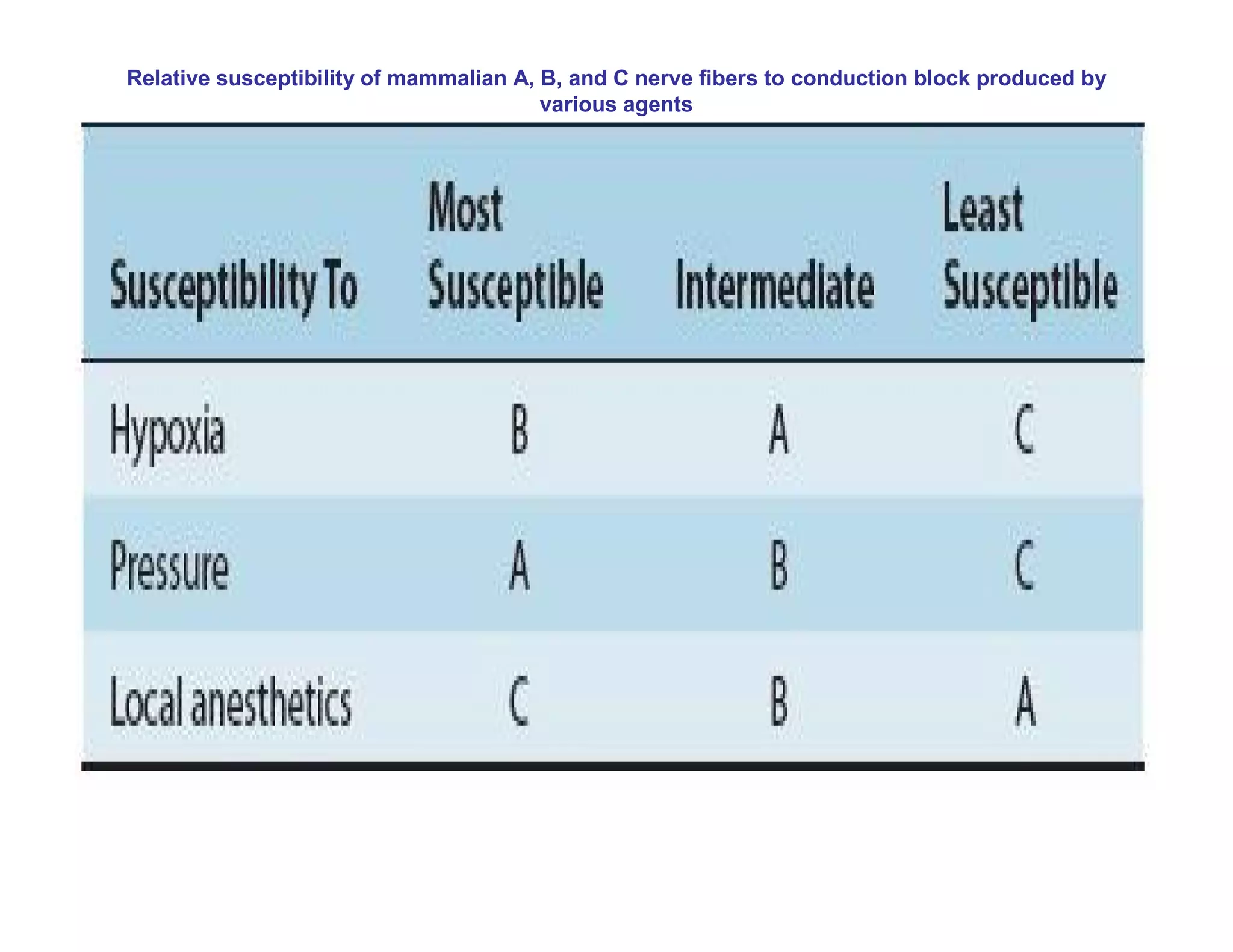
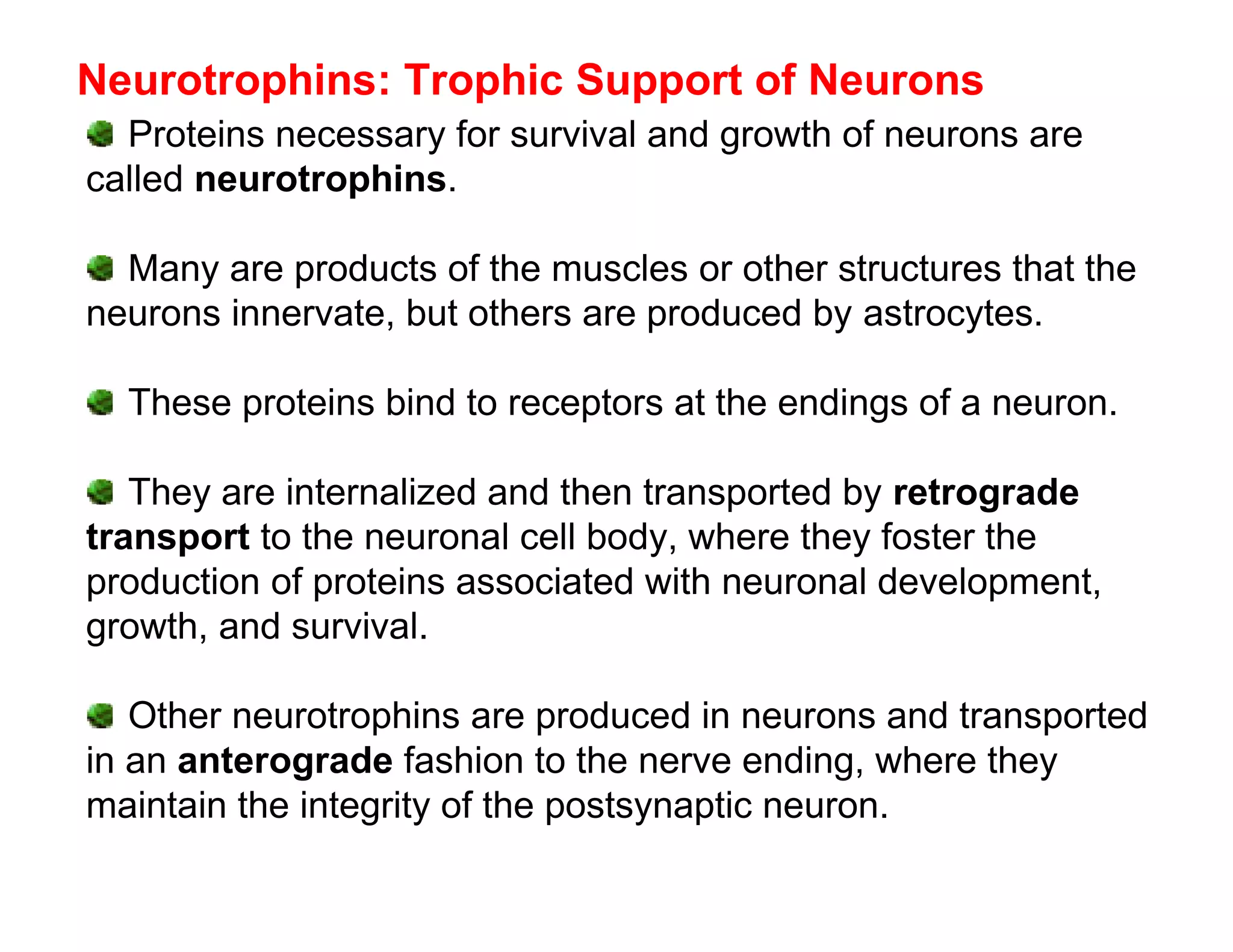
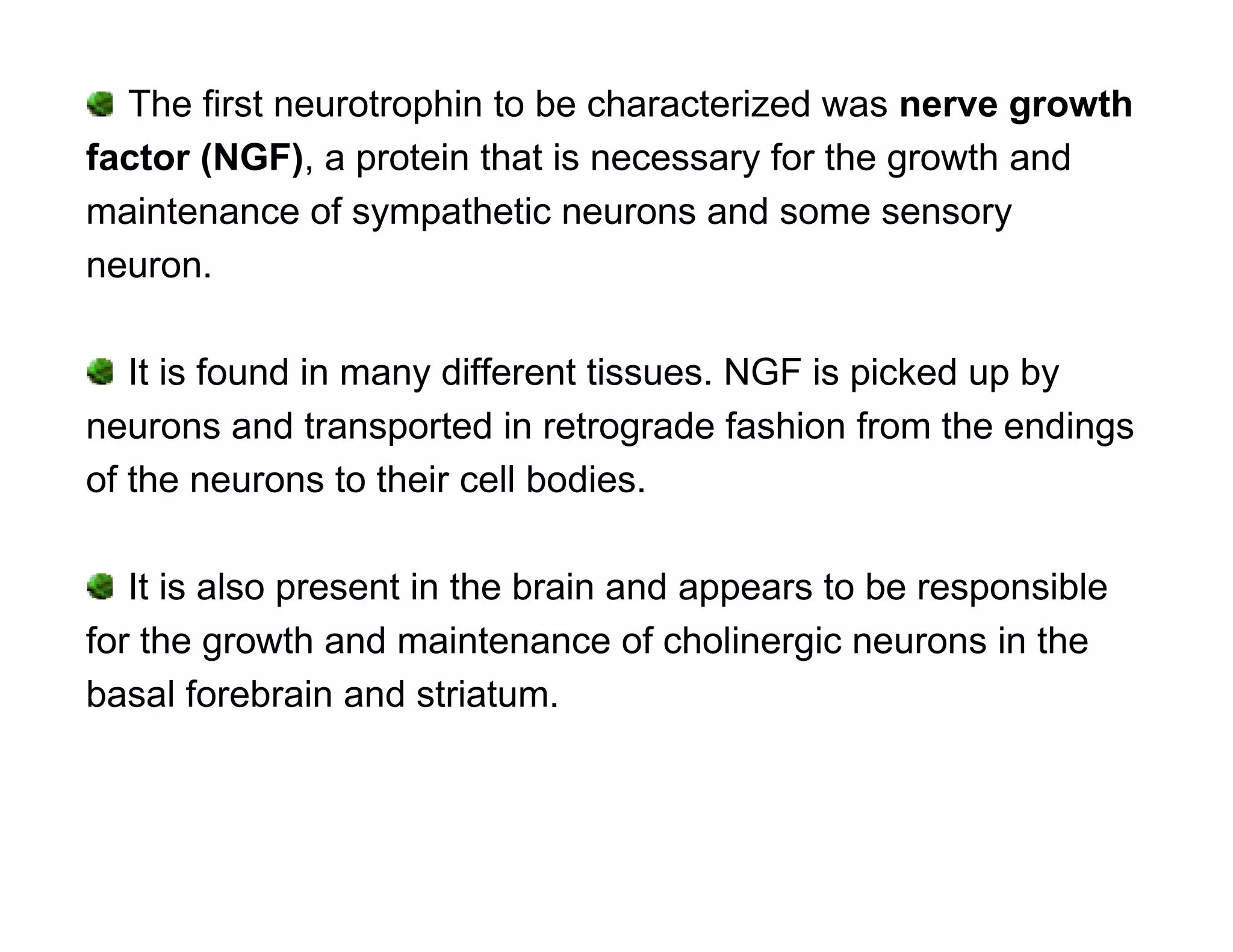
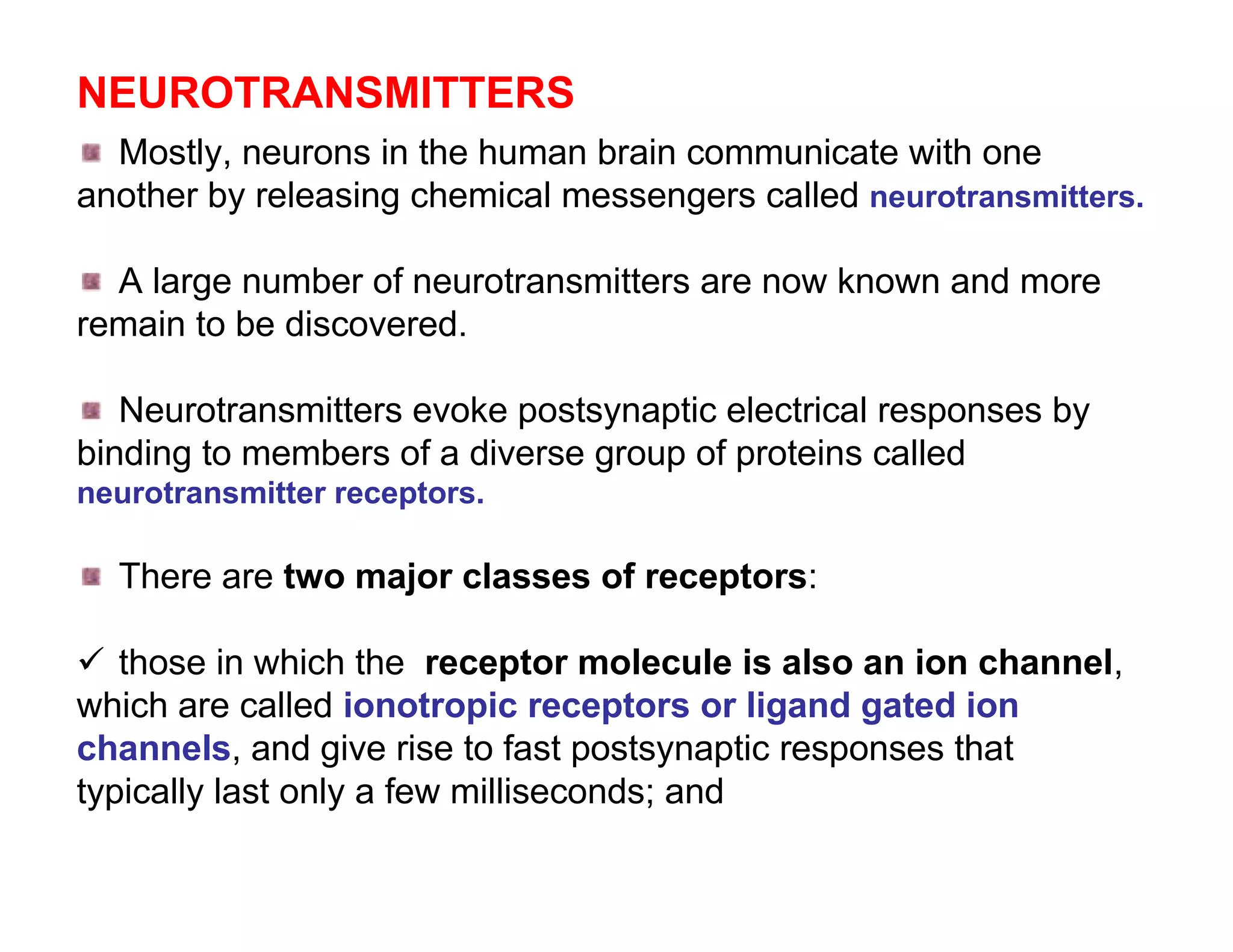
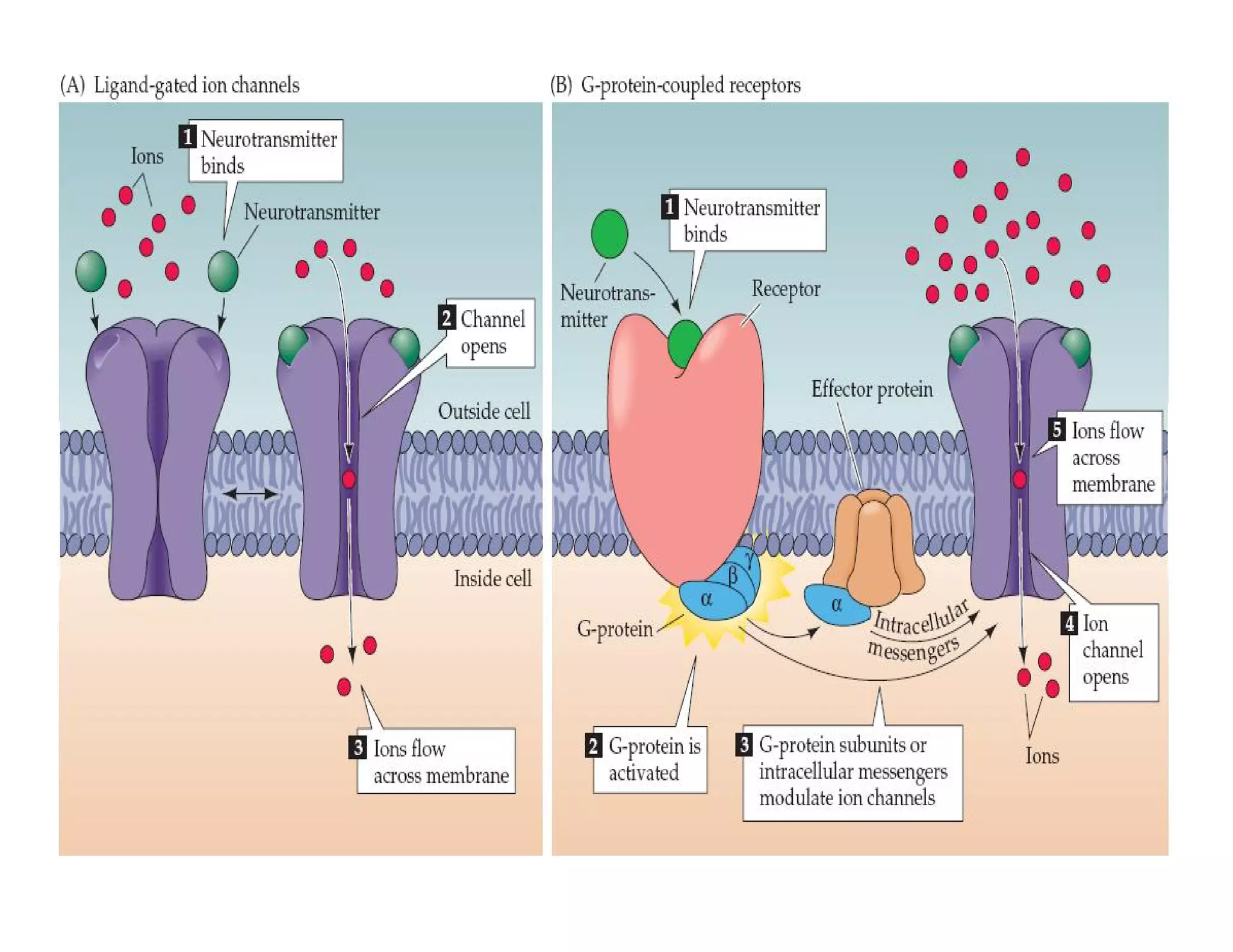
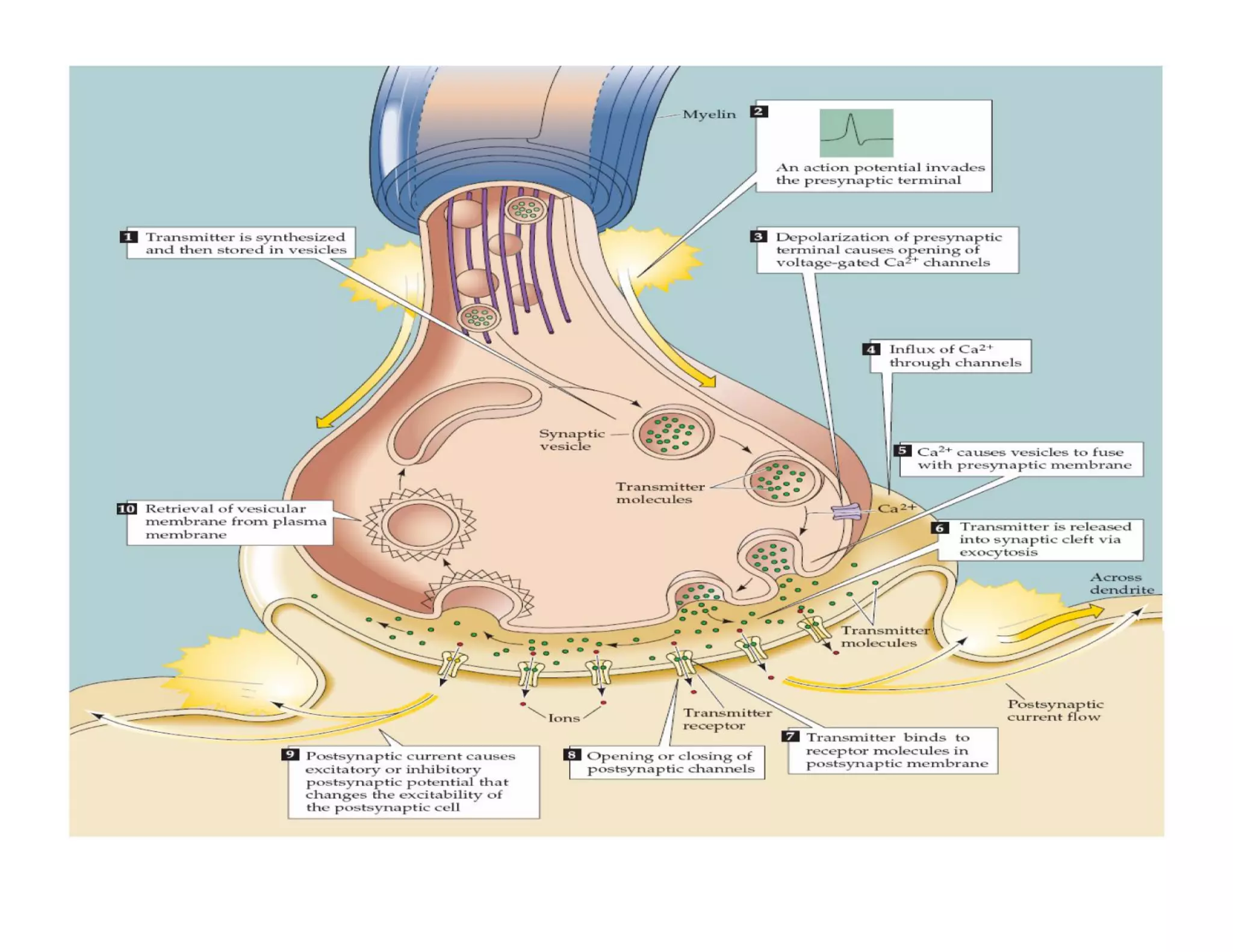
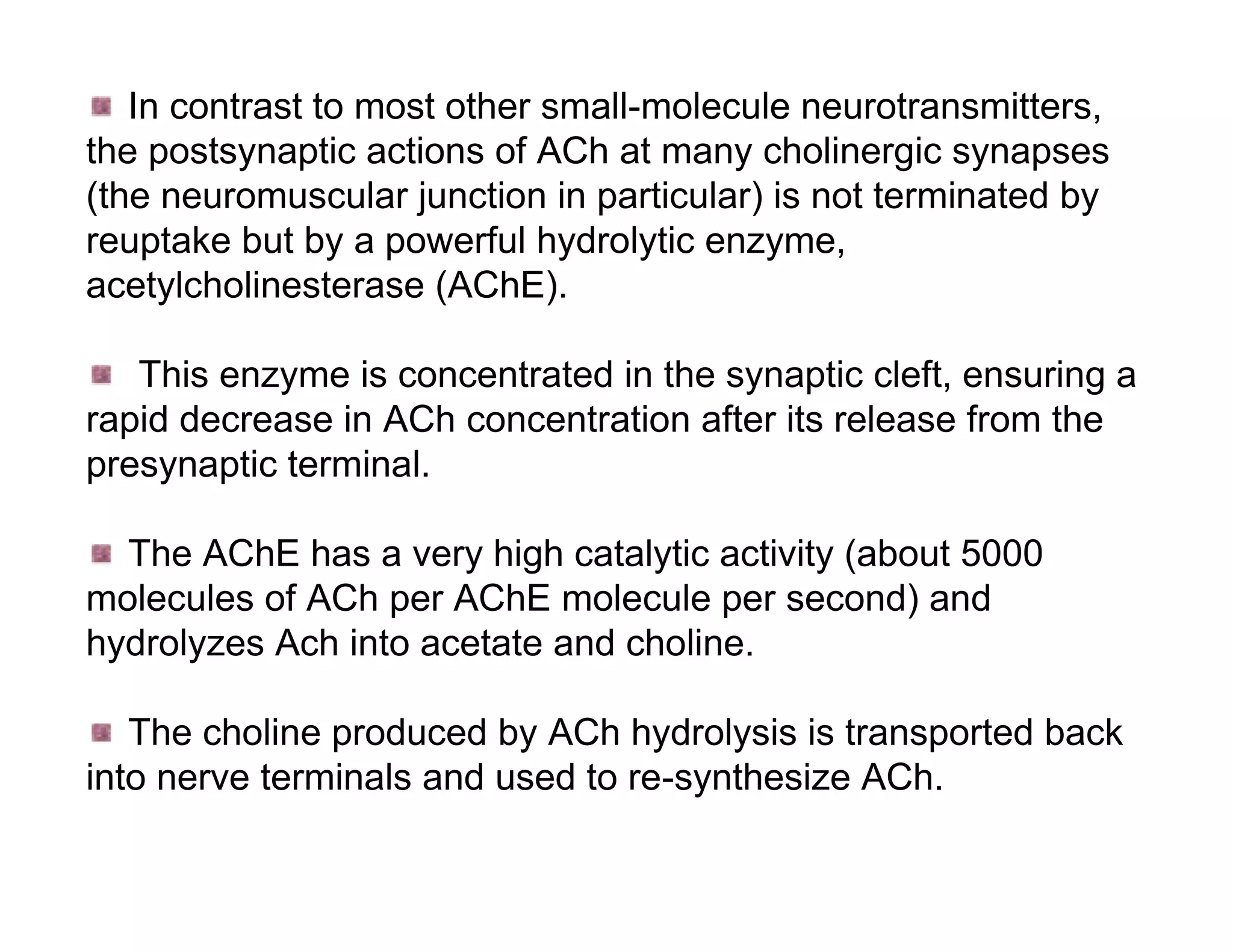
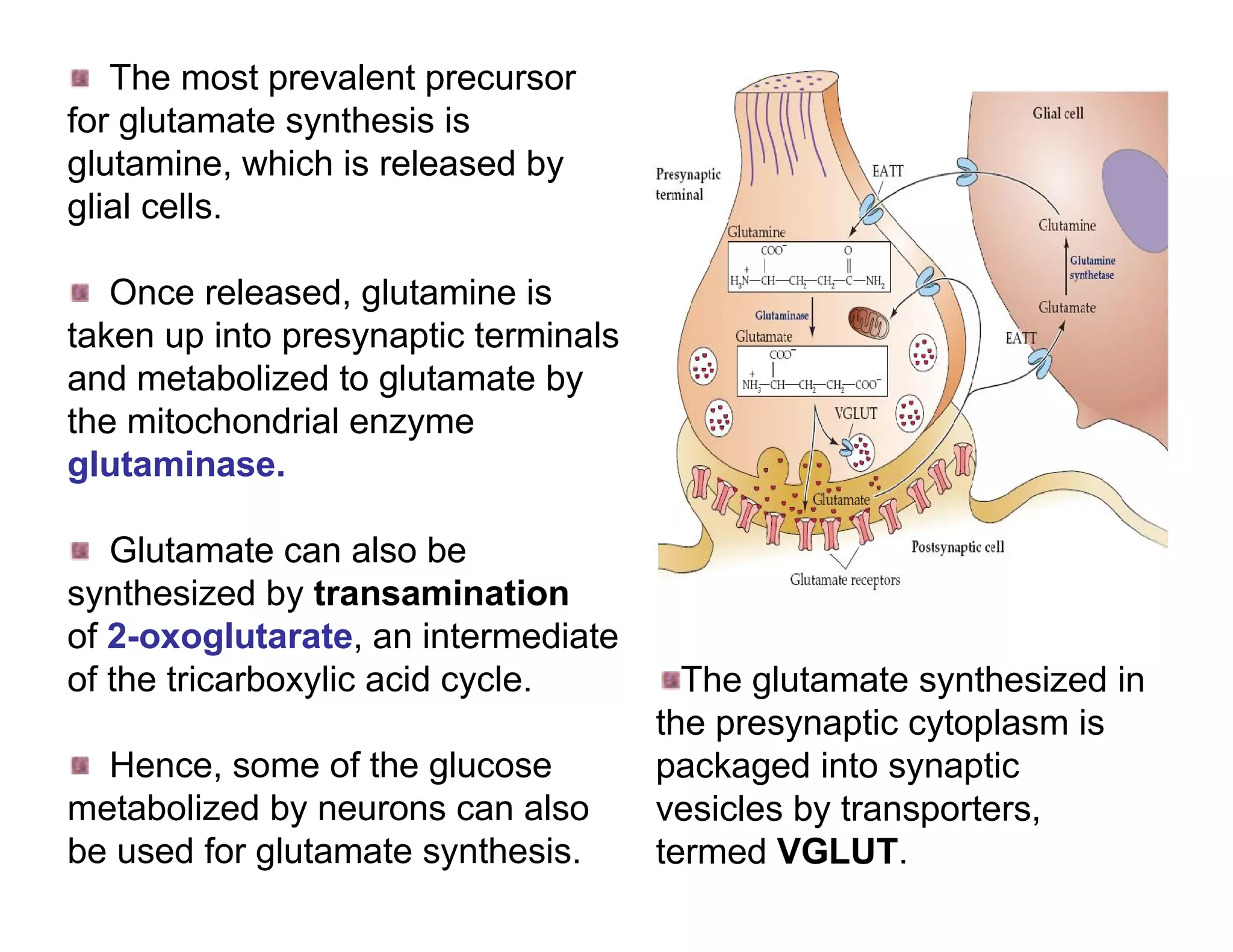
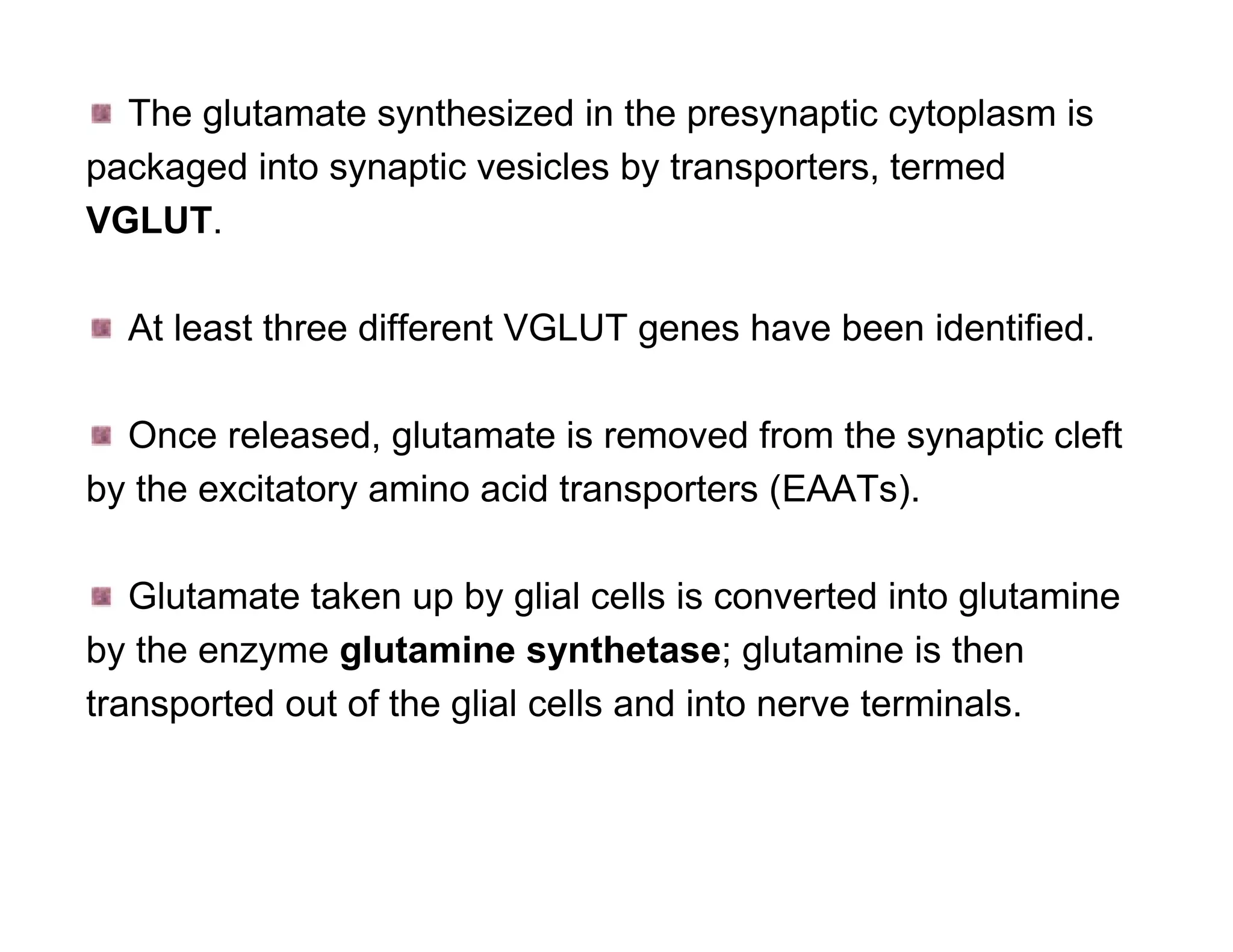
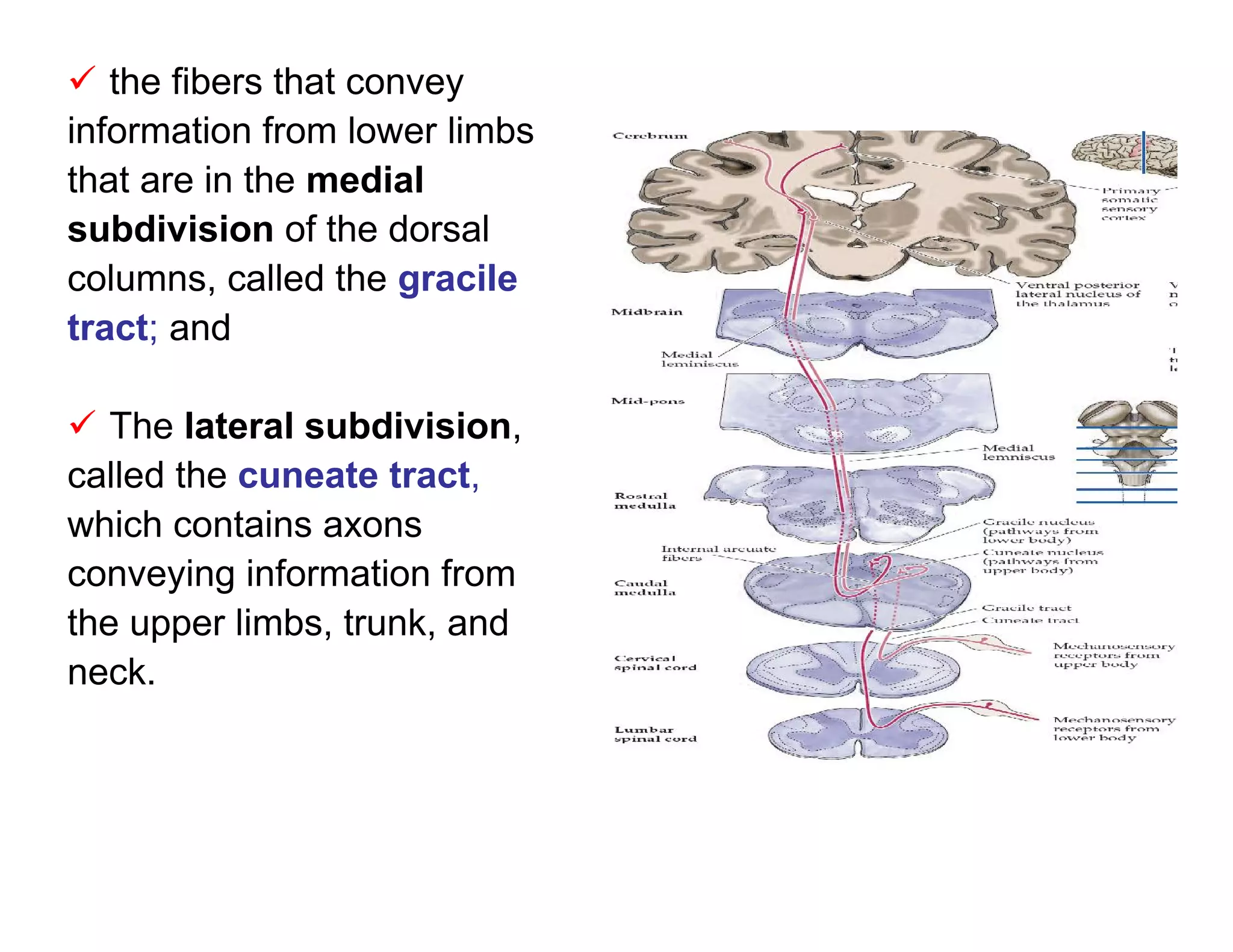
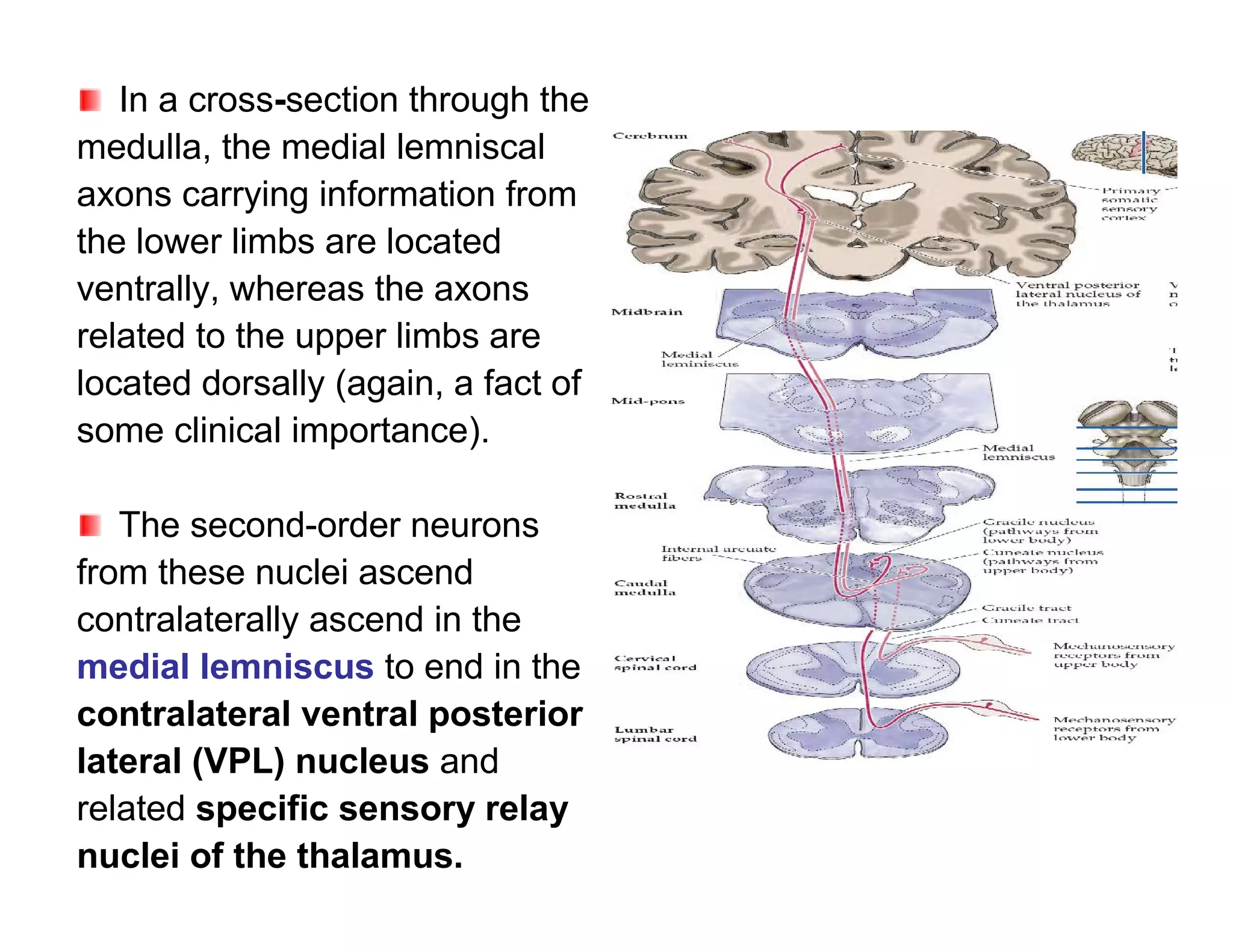
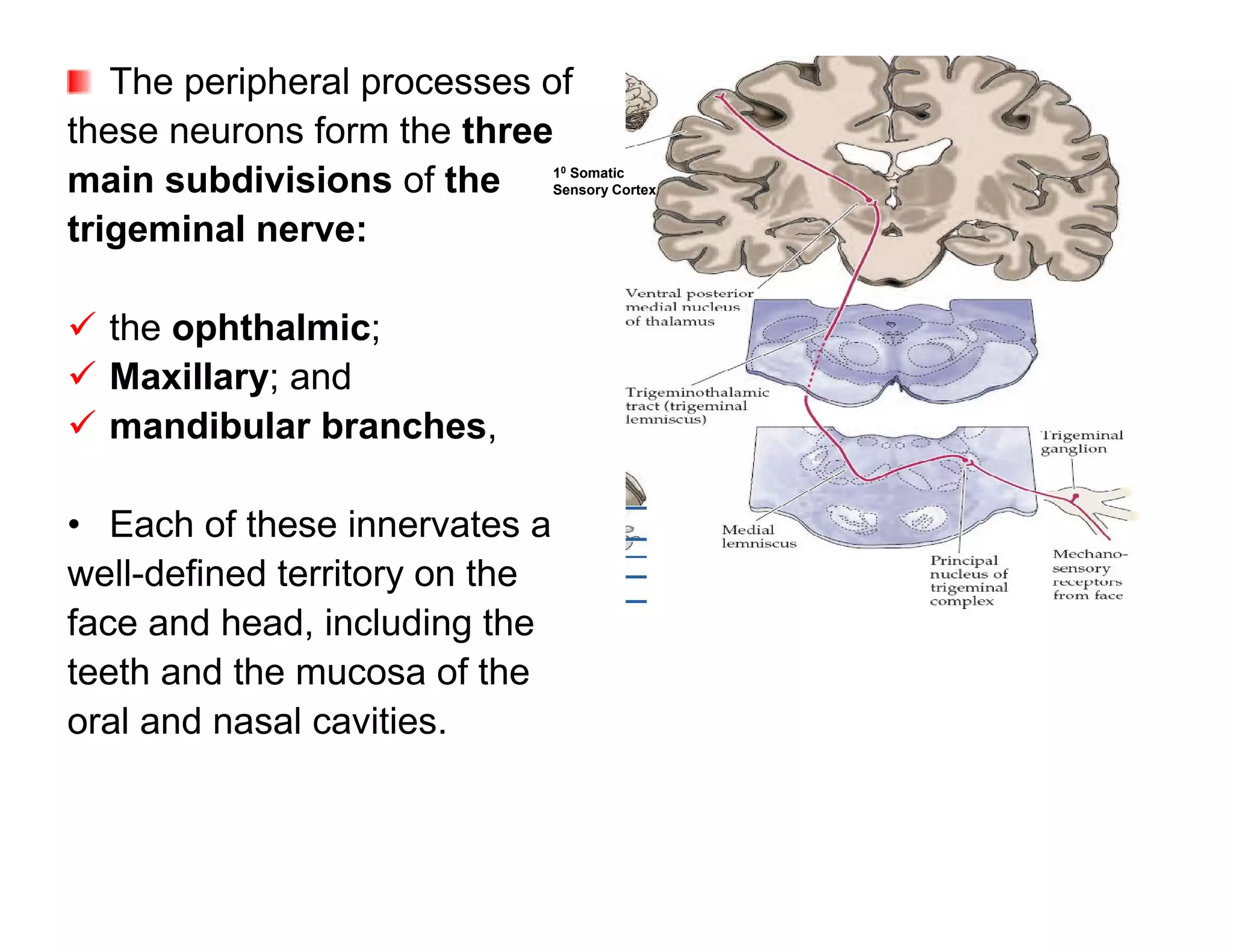
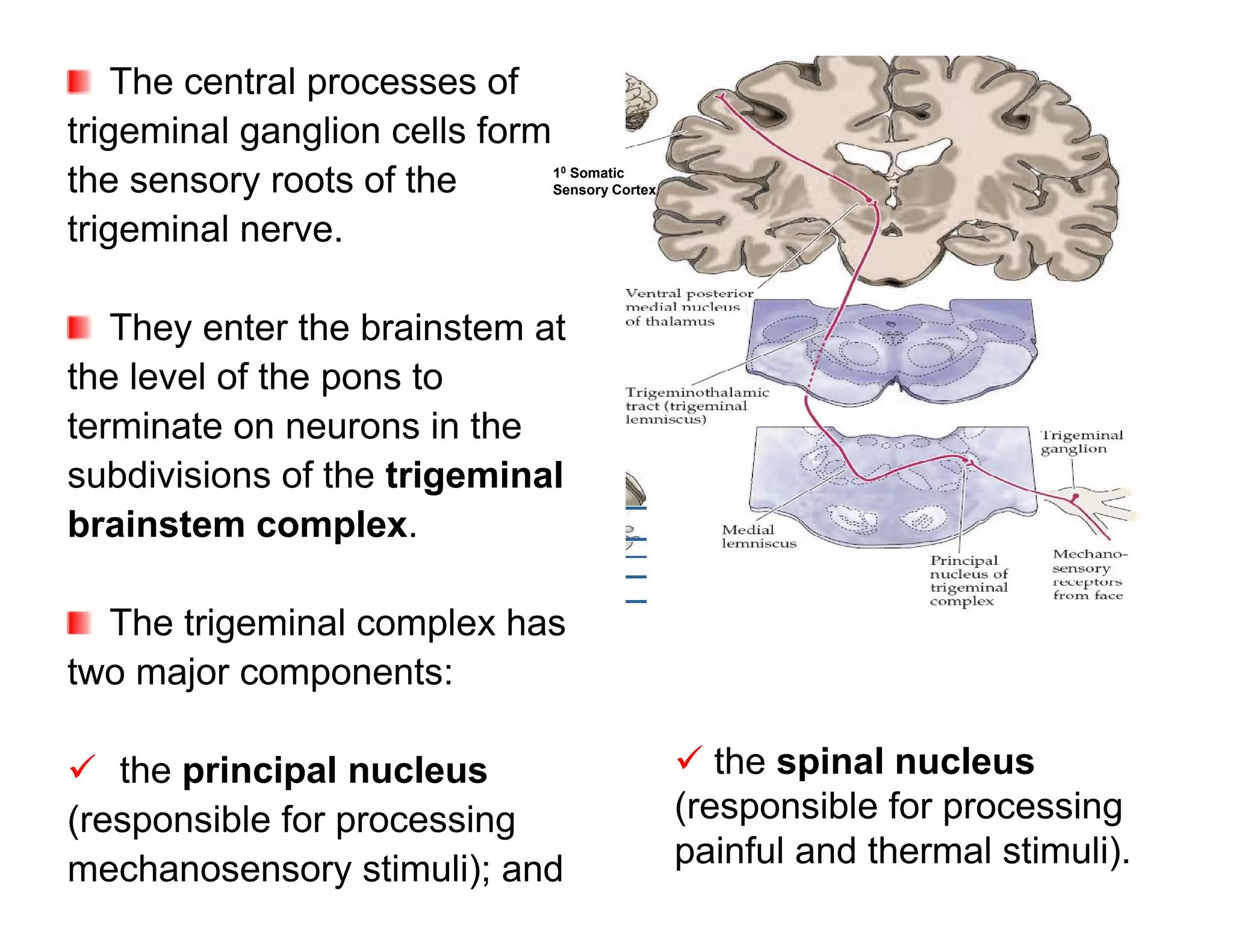
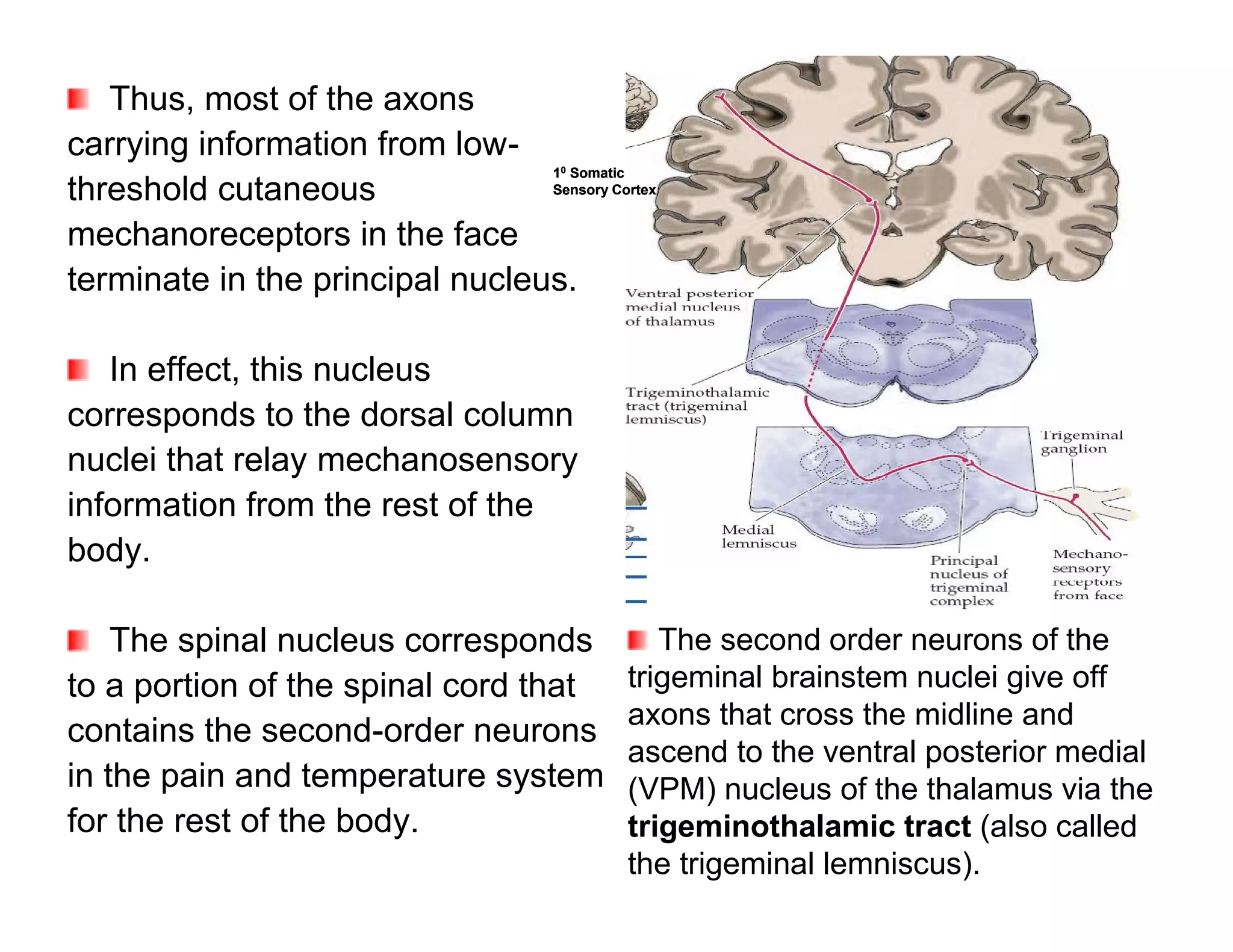
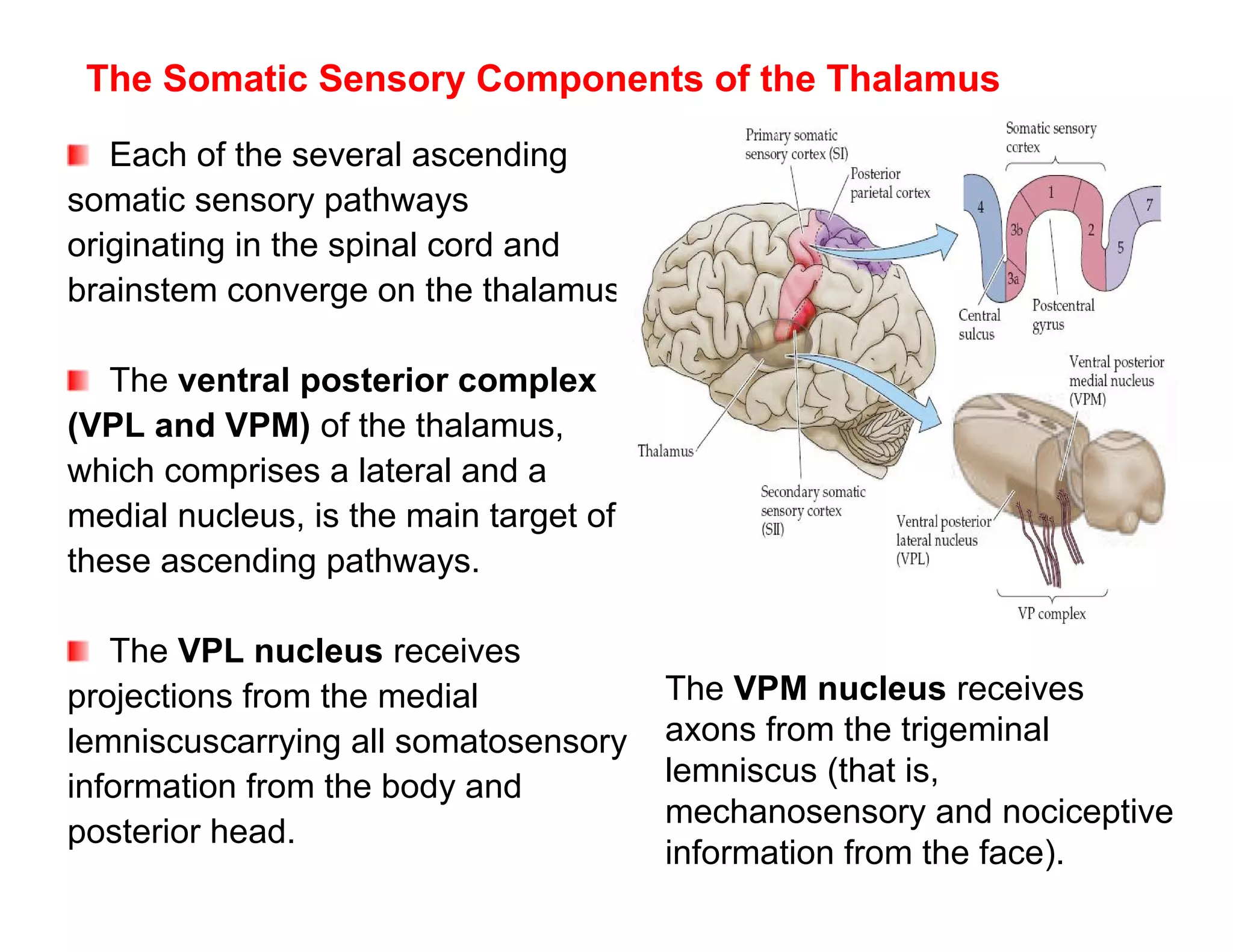
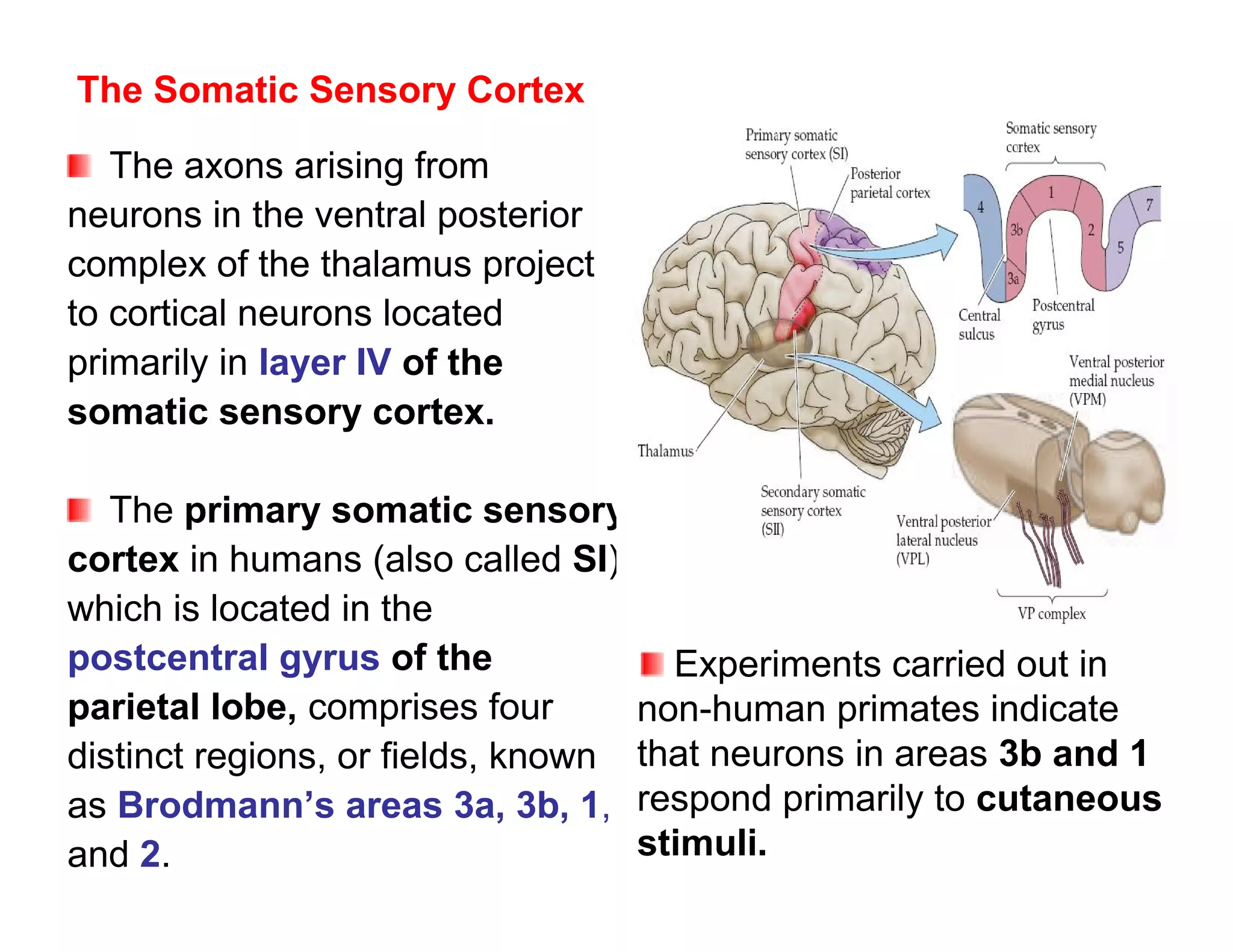
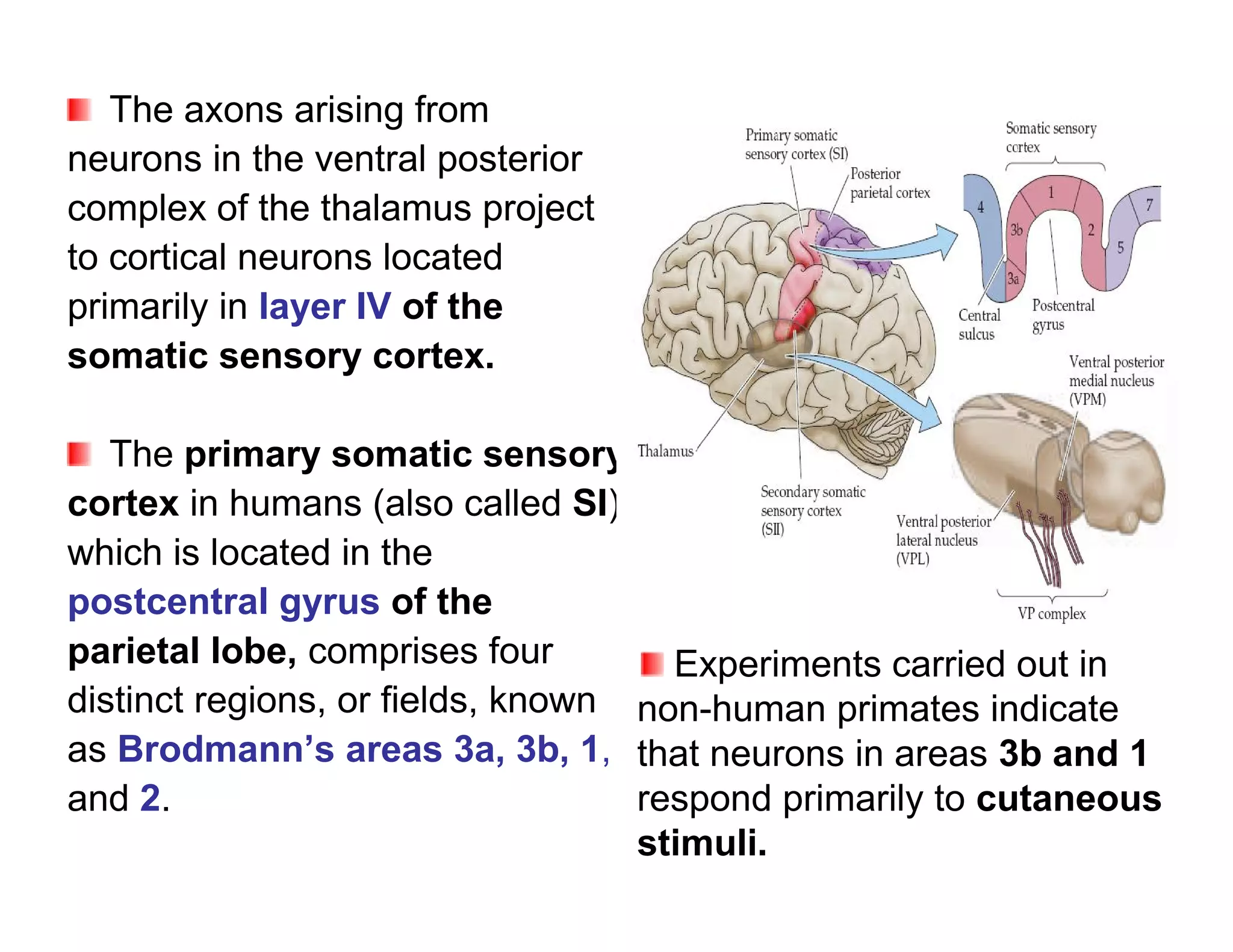
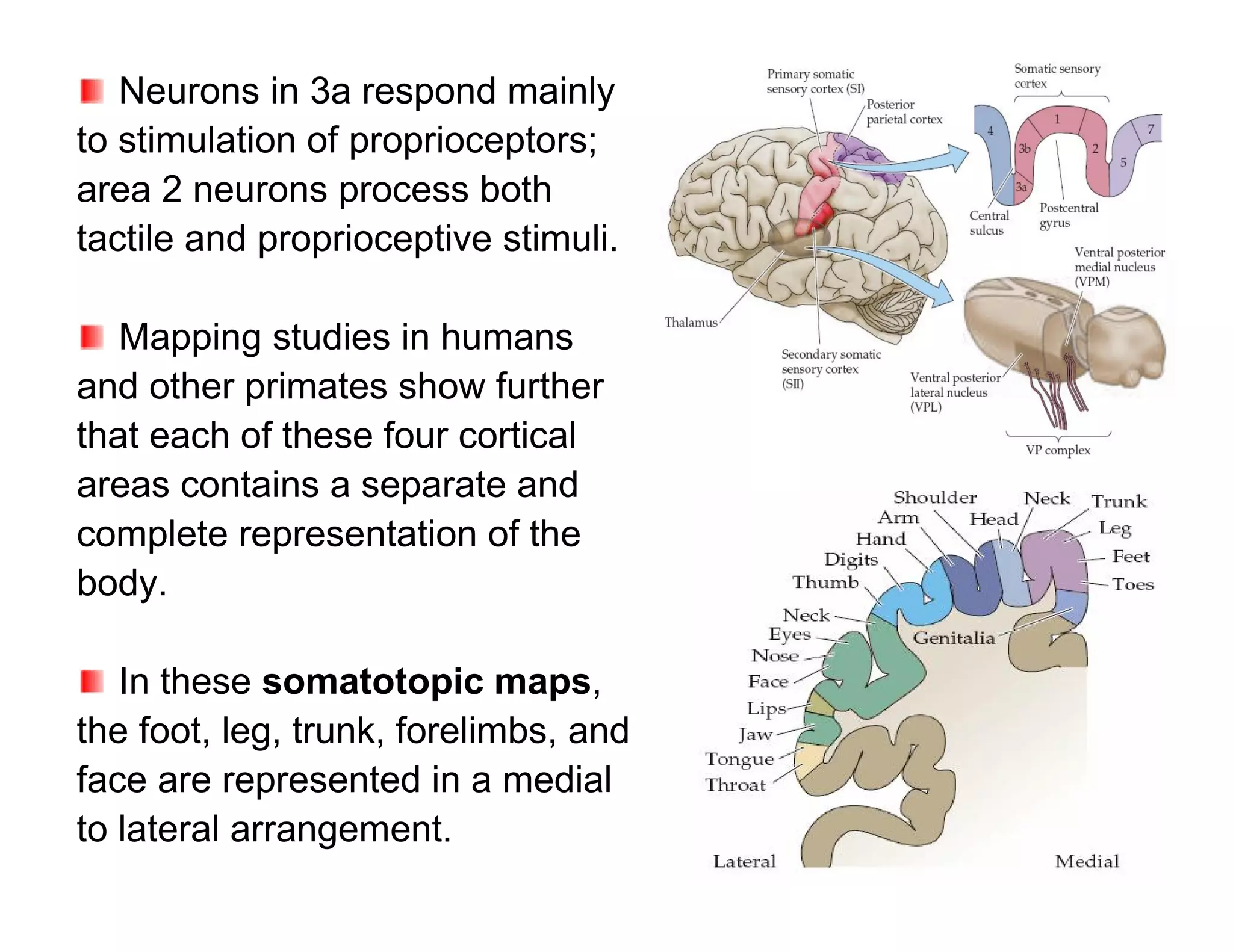
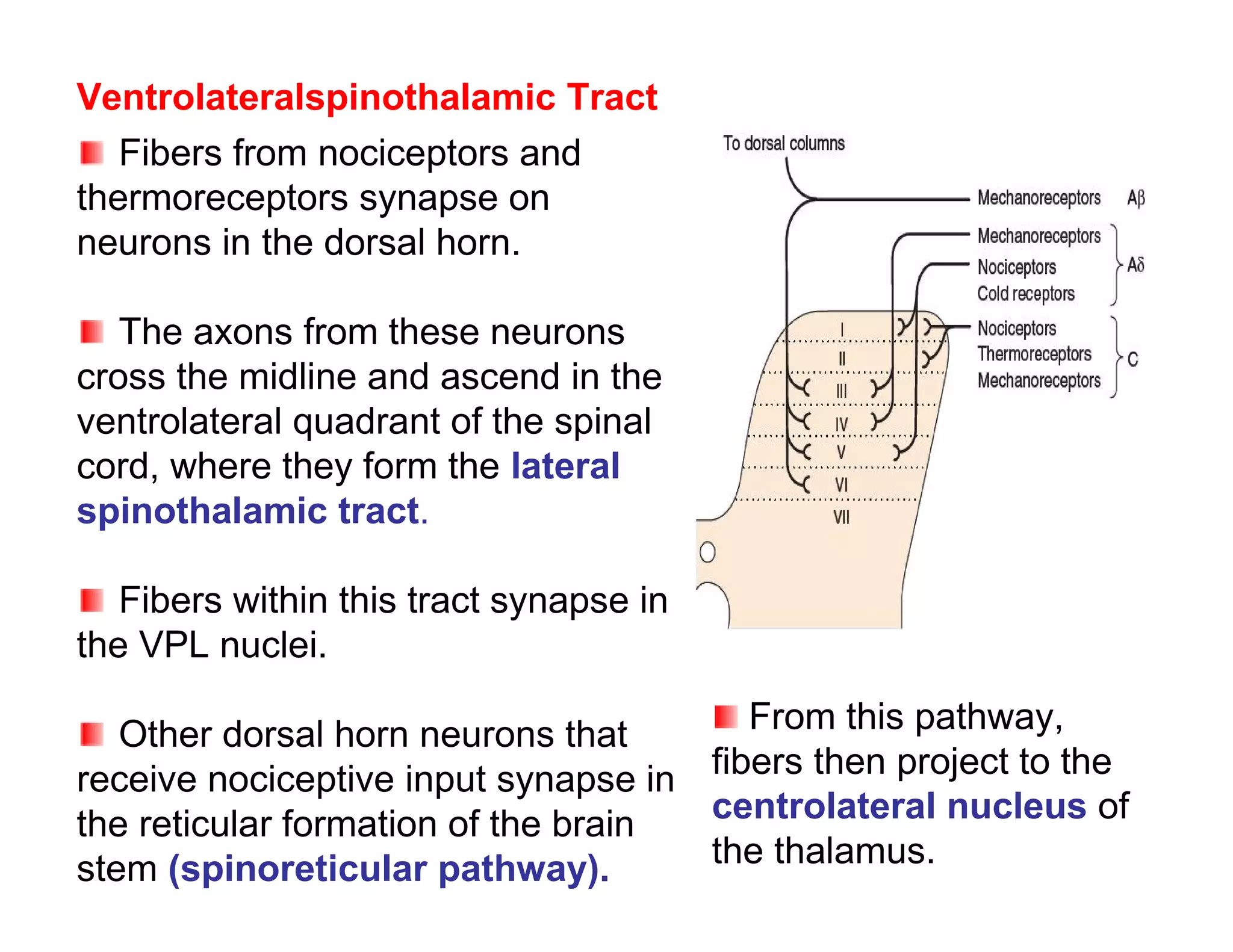
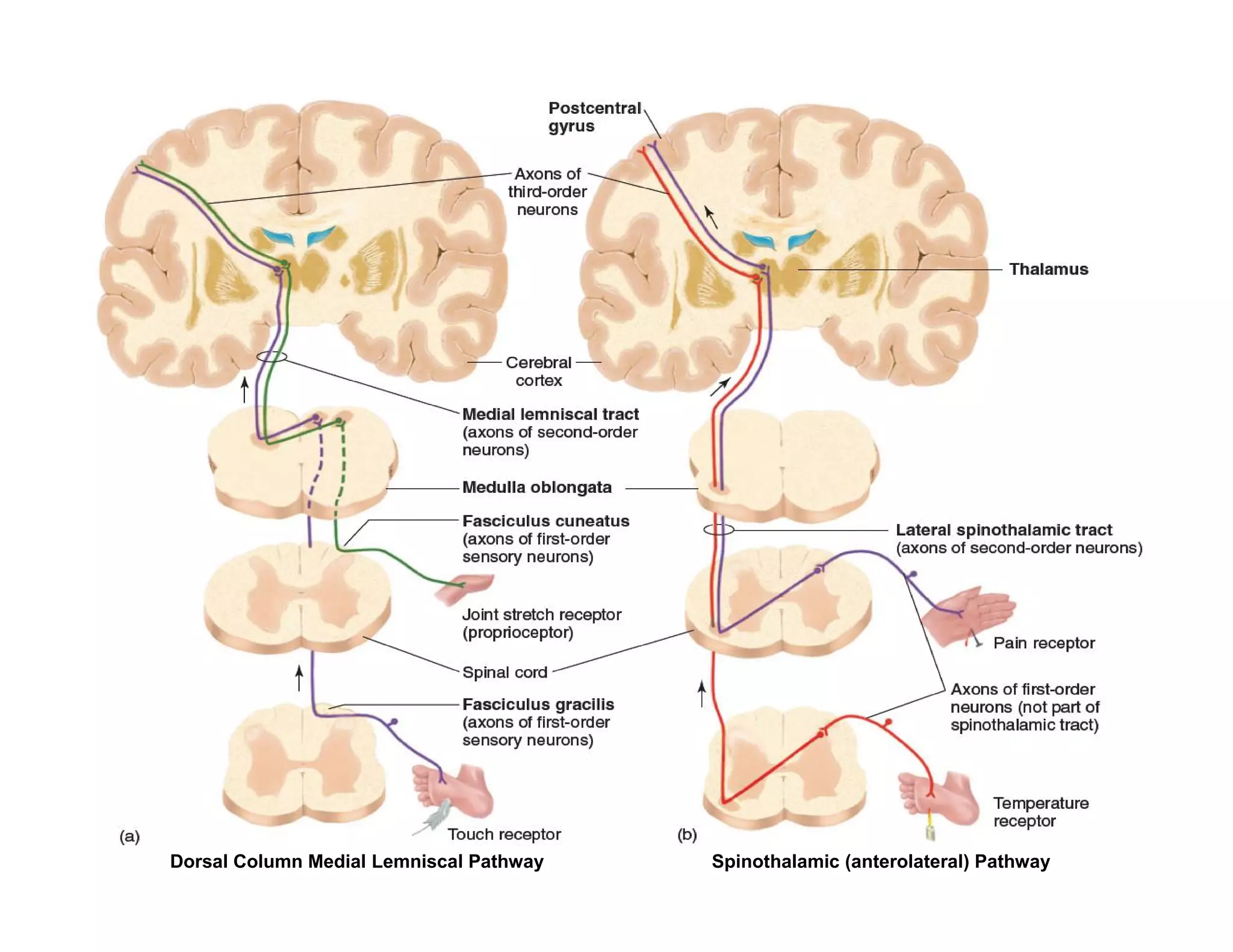
The document provides an overview of neurophysiology and the structure and function of the nervous system. It describes the development of the central nervous system from the neural tube, and defines the major subdivisions of the brain and spinal cord. It also discusses the types of neurons and glial cells, their roles, and the protective mechanisms of the central nervous system like the meninges and blood-brain barrier.























































![In response to tissue injury Injured cells release
chemical mediators can chemicals such as K+ that
sensitize and activate depolarize nerve terminals,
nociceptors thereby making nociceptors more
contributing to hyperalgesia responsive.
and allodynia.
Injured cells also release
bradykinin and substance P,
which can further sensitize
nociceptive terminals.
Other Chemicals include:
histamine is released from
mast cells;
serotonin (5-HT) from
platelets;
Adopted from Kandel ER, Schwartz JH, Jessell TM [editors]:
Principles of Neural Science. McGraw-Hill, 2000.](https://image.slidesharecdn.com/neurophysiologycompletenotehphy3052-121224155008-phpapp02/75/Neurophysiology-complete-note-hphy-305-2-144-2048.jpg)
![calcitonin gene-related peptide All these chemicals
(CGRP) from nerve terminals; and contribute to the
inflammatory process and
prostaglandins from cell activating or sensitizing the
membranes. nociceptors.
Substance P acts on mast
cells to cause degranulation
and release of histamine,
which activates nociceptors
and plasma extravasation
CGRP dilates blood
vessels that together with the
plasma extravasation result
in edema formation.
Adopted from Kandel ER, Schwartz JH, Jessell TM [editors]:
Principles of Neural Science. McGraw-Hill, 2000.](https://image.slidesharecdn.com/neurophysiologycompletenotehphy3052-121224155008-phpapp02/75/Neurophysiology-complete-note-hphy-305-2-145-2048.jpg)
![The resulting edema causes Some released
additional release of bradykinin. substances act by releasing
another one (e.g., bradykinin
activates both Aδ and C
fibers and increases
synthesis and release of
prostaglandins).
Prostaglandin E2 (a
cyclooxygenase metabolite
of arachidonic acid) is
released from damaged cells
and produces hyperalgesia.
This is why aspirin and
other NSAIDs (inhibitors of
cyclooxygenase) alleviate
Adopted from Kandel ER, Schwartz JH, Jessell TM [editors]: Pain.
Principles of Neural Science. McGraw-Hill, 2000.](https://image.slidesharecdn.com/neurophysiologycompletenotehphy3052-121224155008-phpapp02/75/Neurophysiology-complete-note-hphy-305-2-146-2048.jpg)