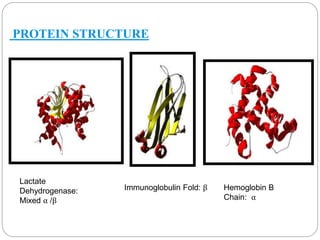
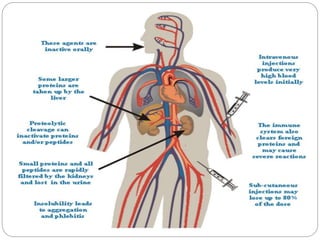
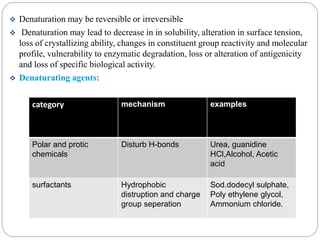
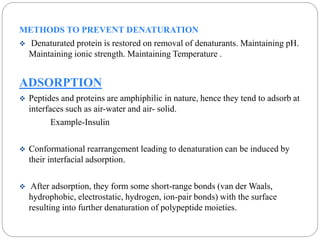
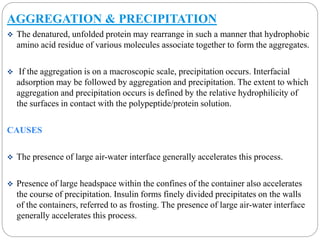
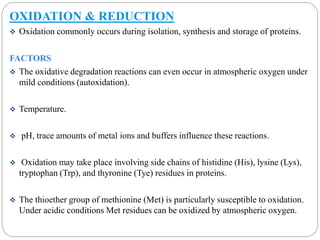
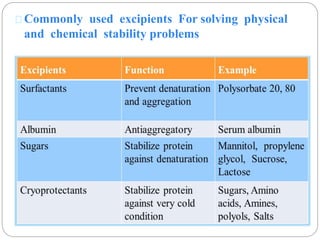
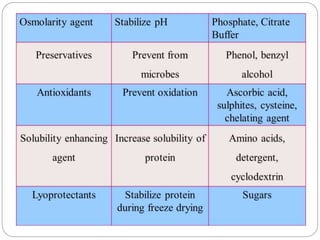
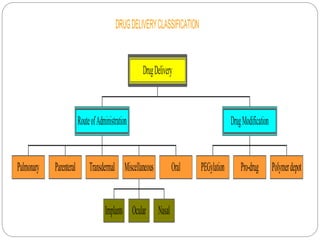
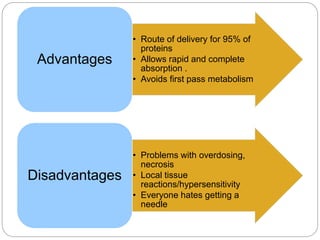
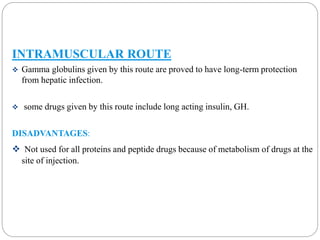
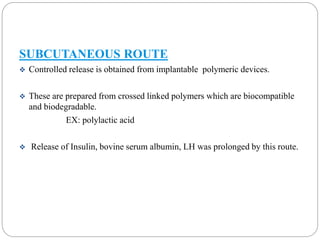
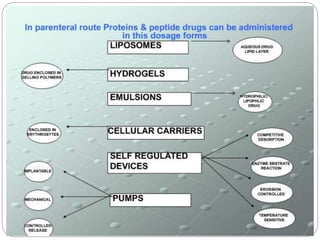
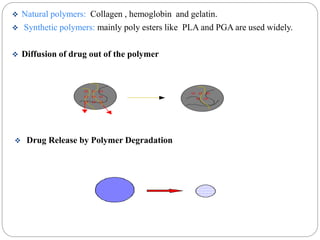
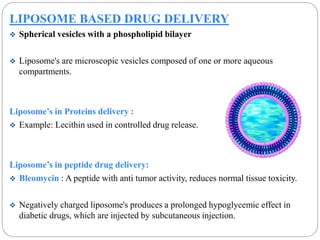
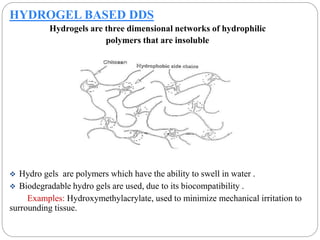
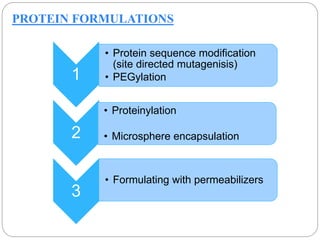
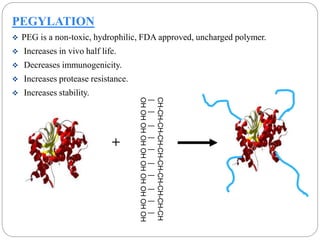
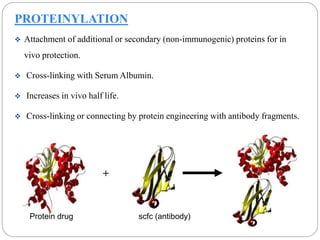
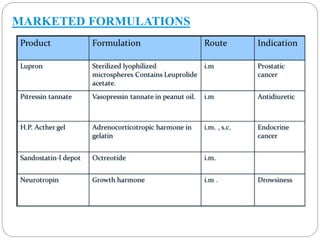
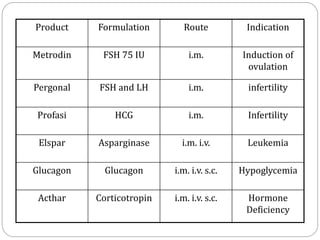
This document discusses protein and peptide drug delivery systems. It begins by defining proteins and peptides, and describing their structures. It then discusses various challenges in delivering protein and peptide drugs, such as stability issues like denaturation, aggregation, oxidation, and proteolysis. It also categorizes different drug delivery routes like parenteral, pulmonary, transdermal, and oral. Finally, it provides examples of marketed protein and peptide drug formulations and discusses strategies to improve stability and delivery of these drugs.