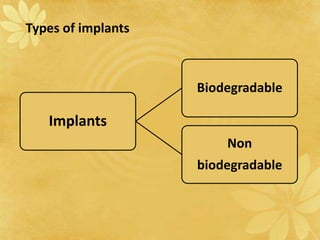
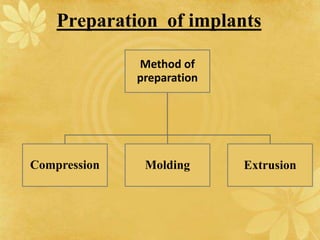
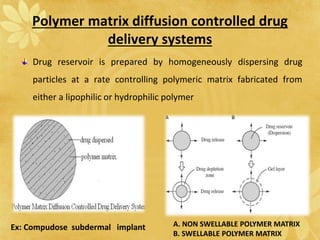
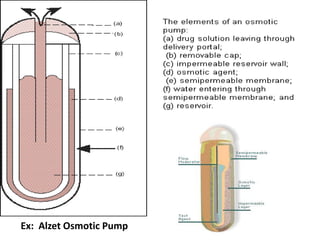
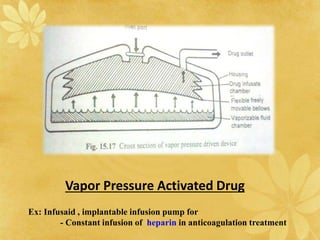
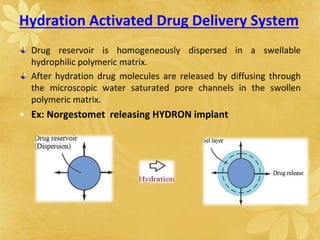
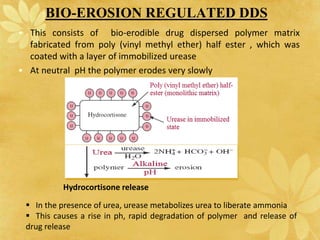
This document discusses implantable drug delivery systems. Implants provide controlled delivery of drugs over long periods of time at the site of implantation. There are biodegradable and non-biodegradable implants. Implants can be classified based on their release mechanism, such as membrane permeation controlled, matrix diffusion controlled, or activation modulated systems. Implants offer benefits like continuous drug delivery and avoidance of peak concentrations but have disadvantages like requiring surgery and host reactions. Common applications of implants include cancer treatment, contraception, and ocular diseases.