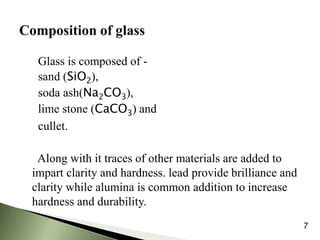
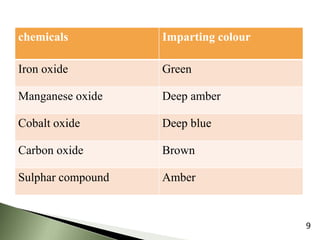
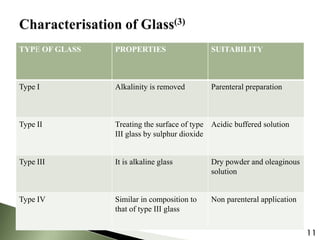
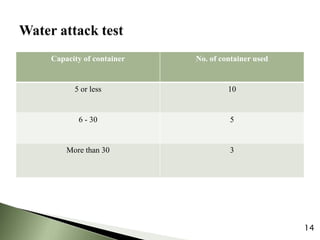
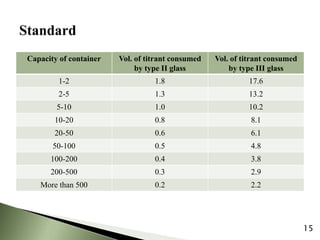
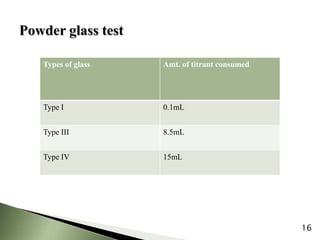
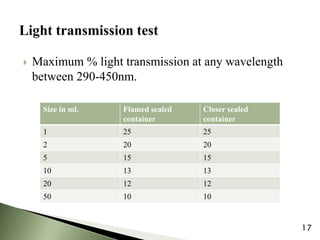
The document provides an overview of pharmaceutical containers, emphasizing the importance of proper packaging for drug stability and storage. It details the types of glass used in container manufacturing, their compositions, and the advantages and disadvantages of using glass as packaging material. Additionally, it outlines various tests for evaluating glass containers and their suitability for different pharmaceutical applications.
![1
Mr. Sagar Kishor Savale
[Department of Pharmaceutics]
avengersagar16@gmail.com
2015-2016
Department of Pharmacy (Pharmaceutics) | Sagar savale](https://image.slidesharecdn.com/glass-160516125957/85/Glass-1-320.jpg)