The document provides an in-depth overview of Transdermal Drug Delivery Systems (TDDS), discussing various aspects such as the introduction to TDDS, its components, advantages, and disadvantages. It explains the structure of the skin and the mechanisms involved in drug penetration, including the role of polymer matrices, penetration enhancers, and pressure-sensitive adhesives. Additionally, it compares TDDS with intravenous and oral drug delivery methods, highlighting their effectiveness and limitations.
![Transdermal Drug Delivery System
[TDDS]
06/04/16SAGAR KISHOR SAVALE1](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/75/Transdermal-Drug-Delivery-System-TDDS-1-2048.jpg)

![A] Introduction to TDDS’s-
Management of illness through medication has entered an era of rapid
growth. A variety of means by which drugs are delivered to the human body
for the therapy such as tablets, capsules, injections, aerosols, creams,
ointments, suppositories, liquids etc. are referred as a conventional drug
formulations.
Among many pharmaceutical dosage forms, continuous intravenous
infusion at preprogrammed rate has been recognized as a superior mode of
drug delivery.
At present, the most common form of delivery of drugs is the oral route.
It has the notable advantage of easy administration.
Contd...
06/04/16SAGAR KISHOR SAVALE3](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-3-320.jpg)

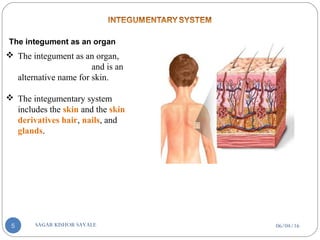





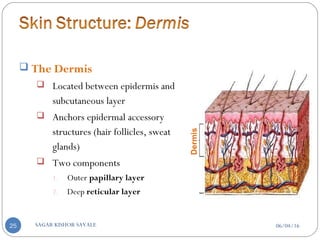
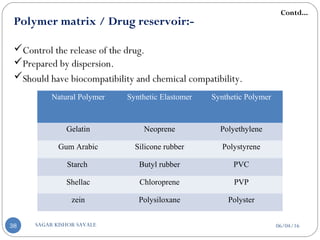
![B] Basic Components Of Transdermal System:-
Polymer matrix / Drug reservoir
Drug
Penetration enhancers
Pressure sensitive adhesive (PSA)
Backing laminates
Release liner
Other excipients like plasticizers and solvents
06/04/16SAGAR KISHOR SAVALE37](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-37-320.jpg)


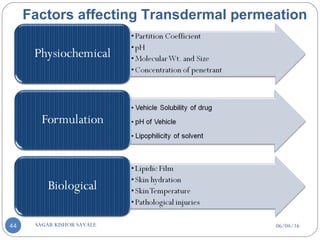
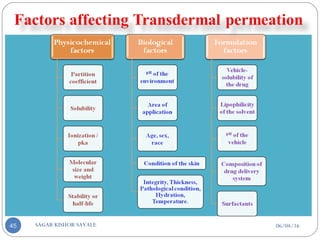
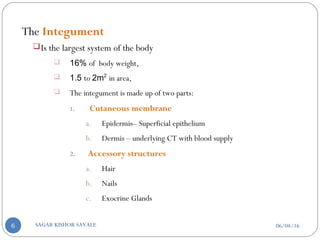
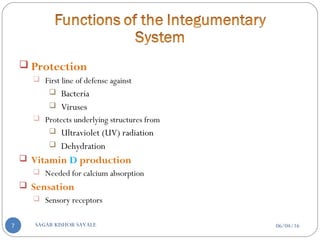
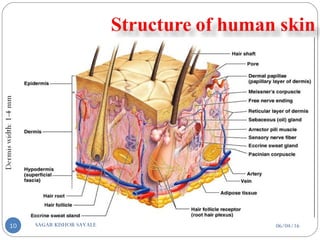
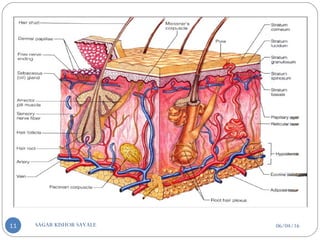
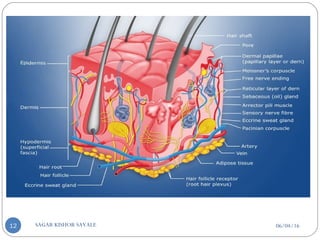
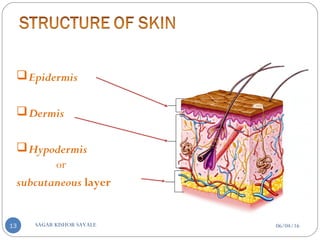
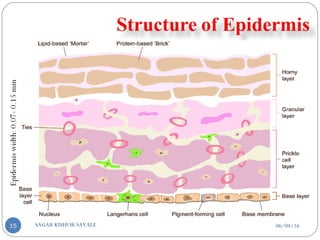
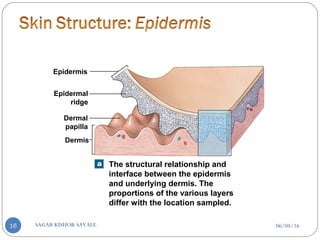
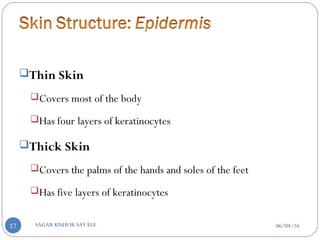
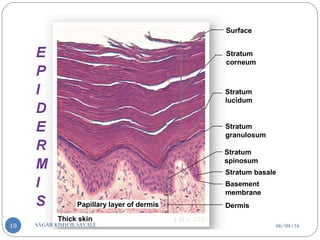
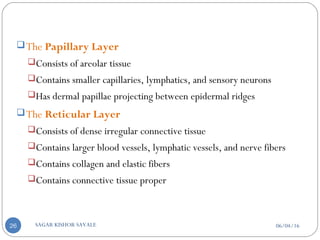
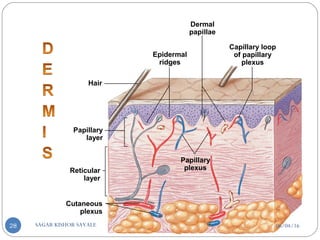
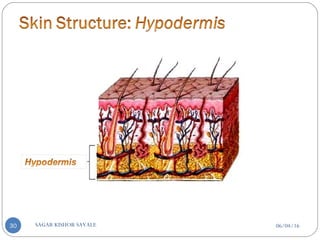
![C] Penetration Enhancement:-
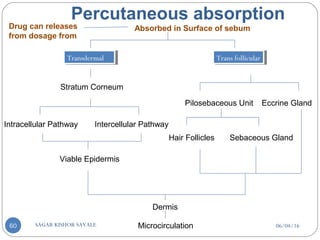
Skin structure as it relates to drug penetration:-
The skin is a multilayered organ, complex in structure and function. It is
composed of-
Outer epidermis (outermost layered called STRATUM CORNEUM)
Viable epidermis (stratum lucidum, stratum granulosum, stratum spinosum and
stratum germinotive)
Dermis
Contd...
06/04/16SAGAR KISHOR SAVALE46](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-46-320.jpg)
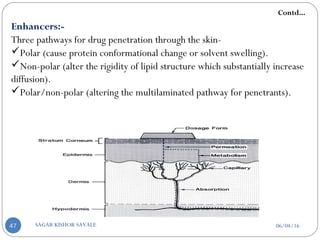
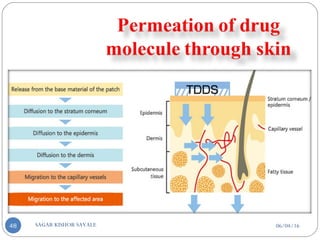
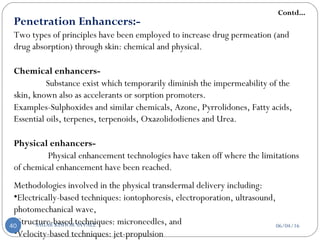
![Classification Of Penetration Enhancement
Chemical Physical
1. Sulphoxides and similar
chemicals
2. Azone
3. Pyrrolidones
4. Fatty acids
5. Essential oils, terpenes
and terpenoids
6. Oxazolidodienes
7. Urea
A] Electrically based techniques-
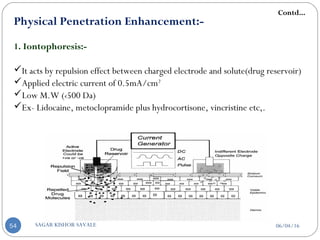
1. Iontophoresis
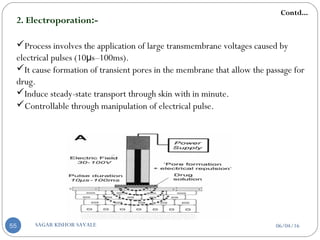
2. Electroporation
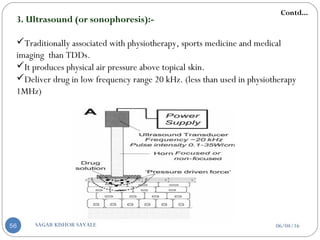
3. Ultrasound (sonophoresis)
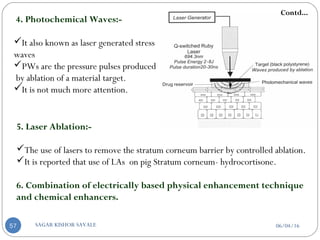
4. Photochemical waves
5. Laser Ablation
6. Combination of electrically based physical
enhancement technique and chemical enhancers.
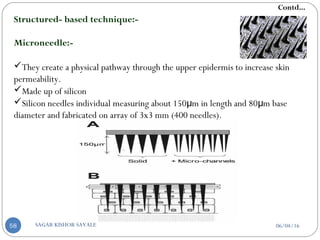
B] Structured based technique-
1. Micro needles
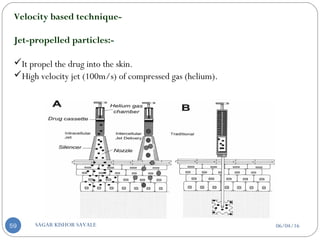
C] Velocity based technique-
1. Jet-propelled particles
Contd...
06/04/16SAGAR KISHOR SAVALE49](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-49-320.jpg)



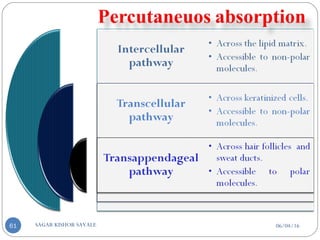


![D] Approaches Used in TDDs:-
Approaches
A) According to drug release
mechanisms:-
1. Matrix-diffusion controlled TDDS’s
2. Membrane-permeation controlled
TDDS’s
3. Microreservoir type or Micro sealed
dissolution controlled TDDS’s
4. Adhesive dispersion type TDDS’s
B) According to rate
controlling step:-
1. Those control the rate
of drug delivery to skin.
2. Those allow the skin to
control the rate of drug
absorption.
C) According to
polymer:-
1. Hydrophilic or
hydro gel system
2. Hydrophobic or
occlusion system
Contd...
06/04/16SAGAR KISHOR SAVALE65](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-65-320.jpg)
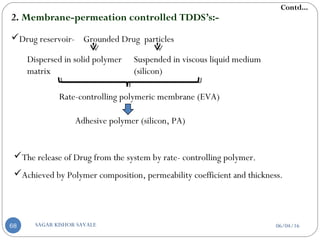
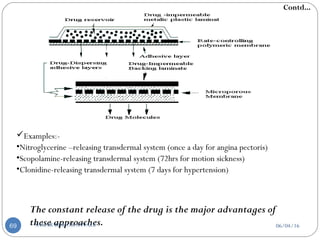
![a] According to drug release mechanisms:-
1. Matrix-diffusion controlled TDDS’s:-
Drug Reservoir- Dispersing drug particles in a hydrophilic or lipophilic
polymer matrix which is accomplished by:
Homogeneously mixing of grounded particles
Liquid
polymer
Highly viscous
base polymer
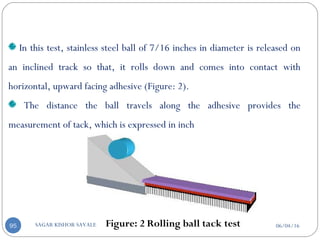
Blending of particle with
rubbery polymer
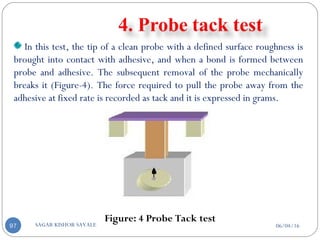
Molded in medicated disc
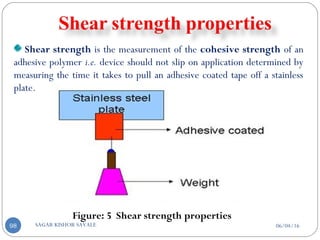
Also by dissolving Drug and Polymer in common solvent solvent
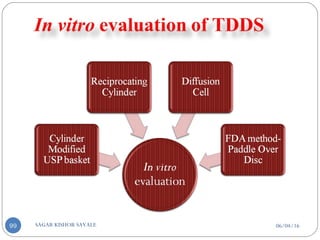
evaporation method
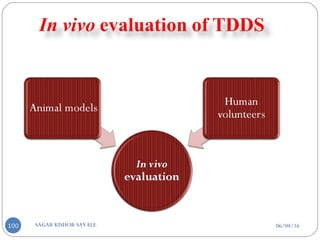
Pasted on occlusive base plate
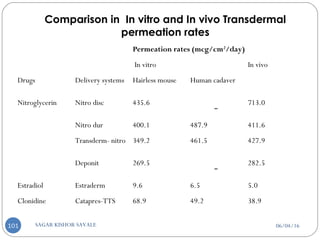
Impermeable plastic membrane
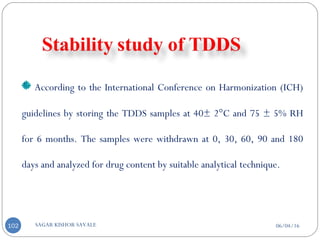
Contd...
06/04/16SAGAR KISHOR SAVALE66](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-66-320.jpg)
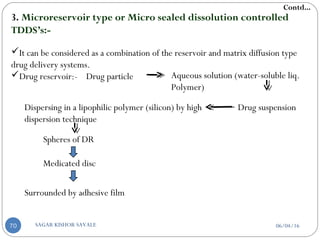
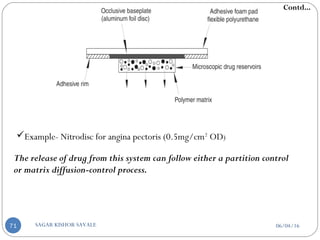
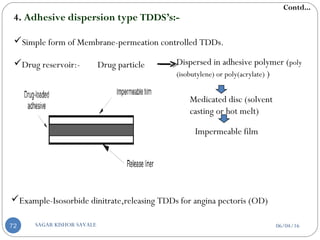
![b] According to rate controlling step:-
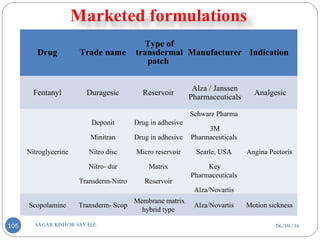
Those they control the rate of drug delivery to the skin.
Potent, for maintaining MEC
Those that allow the skin to control the rate of drug absorption.
Having wide range of plasma drug concentration.
c] According to polymer:-
Hydrophilic or hydro gel system:-
Drug release by swelling mechanism.
Patch of these type absorb water from the skin.
Causes skin irritation & burning sensation.
Contd...
06/04/16SAGAR KISHOR SAVALE73](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-73-320.jpg)

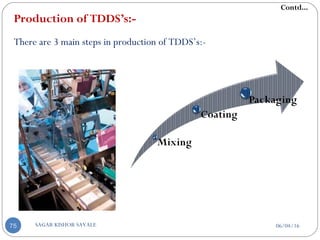
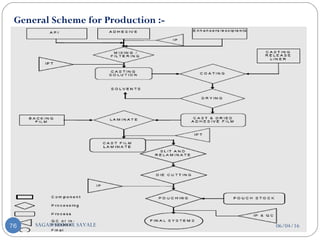
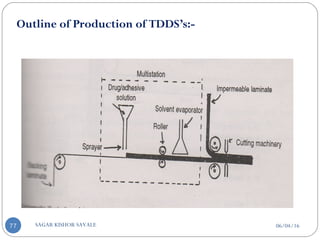
![E] Model Drug Selection Criteria:-
Most drugs are not suitable candidate for Transdermal drug delivery for one or
more reason-
‘Easy to deliver’ drugs have already been commercialized into TDDs.
To date 10 drugs out of 100 have been commercialized into TDDs.
More than 35 patches spanning 13 molecules present in market.
For successfully developing a transdermal drug delivery system, the drug should
be chosen with great care.
The drug possesses the right mix of physicochemical and biological properties
for transdermal drug delivery.
Contd...
06/04/16SAGAR KISHOR SAVALE78](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-78-320.jpg)



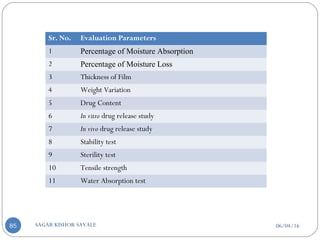
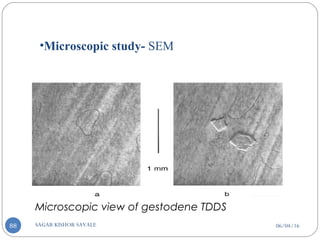

![06/04/16SAGAR KISHOR SAVALE90
Flatness one strip is cut from the centre and two from
each side of patches. The length of each strip is
measured and variation in length is measured by
determining percent constriction. Zero percent
constriction is equivalent to 100 percent flatness.
% constriction =[(I1-I2)/I1]X100
I2 = Final length of each strip
I1 = Initial length of each strip
Percentage of moisture content
% Moisture content = Initial weight – Final weight X
100
Final weight](https://image.slidesharecdn.com/transderaldrugdeliverysystemtdds-160604065142/85/Transdermal-Drug-Delivery-System-TDDS-90-320.jpg)