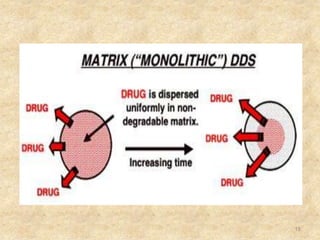
This document discusses sustained release formulations. It defines sustained release as slowly releasing a drug substance from a dosage form over an extended period of time, usually 8-12 hours, to maintain a therapeutic effect. The goals of sustained release formulations are to obtain zero-order drug release, improve patient compliance by reducing dosing frequency, decrease side effects, improve drug utilization, and provide more efficient treatment. The document then discusses techniques for developing sustained release formulations, challenges, and factors to consider like drug properties and the target site of delivery.