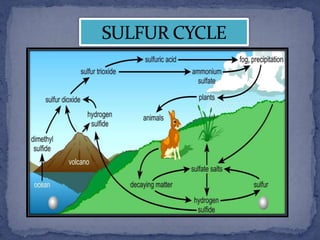
The sulfur cycle describes the movement of sulfur through the biosphere and lithosphere. Sulfur is released into the atmosphere through volcanic eruptions, fossil fuel burning, and decaying organic matter. It is then converted to sulfuric acid and deposited back on land and oceans through precipitation. Sulfur is essential for life and cycles between its reduced and oxidized forms as it moves between living and nonliving parts of the Earth system. Human activities like burning coal have increased sulfur dioxide levels in the atmosphere and contributed to acid rain formation.