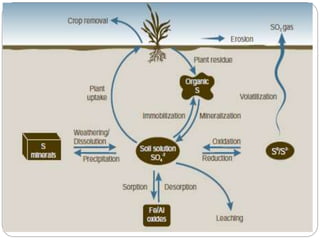
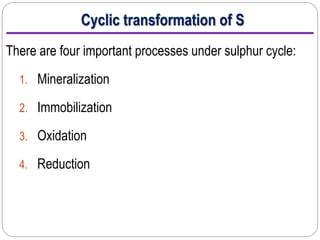
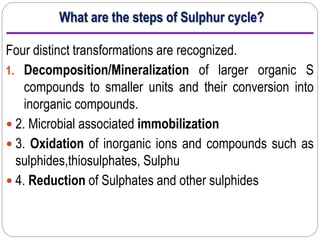
This document provides information on the sulfur cycle. It discusses how sulfur is an essential nutrient for plants and occurs naturally in rocks, soil organic matter, and the atmosphere. The sulfur cycle involves four main processes: mineralization, immobilization, oxidation, and reduction. Sulfur cycles through these processes as it is released from rocks through weathering, taken up by plants and microbes, consumed by animals, and released again through decomposition. Key microbial actors like Thiobacillus bacteria are involved in oxidizing inorganic sulfur compounds in both aerobic and anaerobic conditions. The cycling of sulfur makes both organic and inorganic forms available to support life.