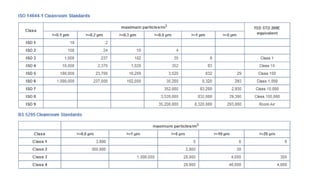
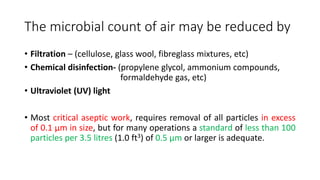
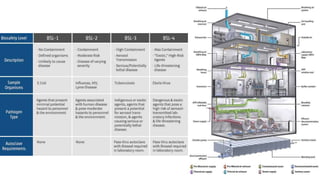
Microbial contamination of pharmaceutical products can be influenced by the environment, materials, formulation, equipment, and personnel involved in production. Good manufacturing practices, hazard analysis, and cleanroom facilities are important to minimize contamination. Strict control of air quality, water sources, packaging, and equipment is necessary, as microorganisms may be introduced from these sources or survive and grow if conditions allow. Proper cleaning and disinfection of equipment, facilities, and raw materials is essential to control microbial contamination in pharmaceutical manufacturing.