Embed presentation
Download to read offline

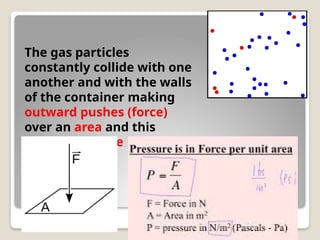
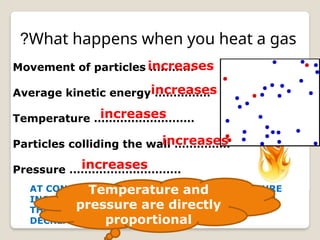

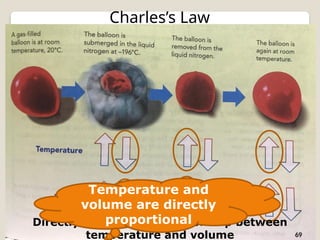
The document discusses the behavior of gases, focusing on how gas particles collide with each other and their container, which generates pressure. It explains that as temperature increases, pressure also increases at constant volume, highlighting Charles's Law, which describes the direct proportionality between temperature and volume. Additionally, it mentions Boyle's Law, illustrating the inverse relationship between volume and pressure.





