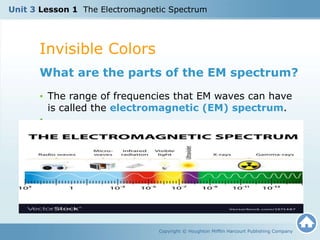
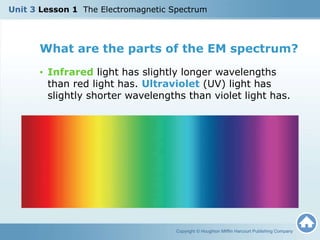
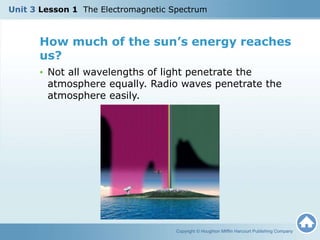
The document discusses the electromagnetic spectrum and the nature of light. It explains that light waves are electromagnetic waves that transfer energy, and that all electromagnetic waves travel at the speed of light. It describes how different wavelengths of light correspond to different colors and are perceived by the human eye. The document also discusses how the electromagnetic spectrum includes invisible wavelengths like infrared and ultraviolet light, and how different wavelengths penetrate the atmosphere and can be harmful at high frequencies.