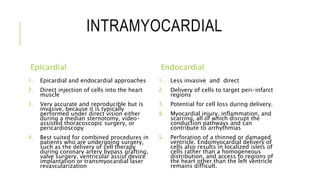
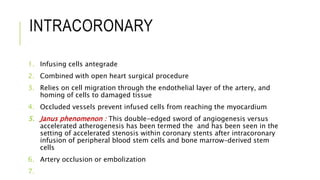
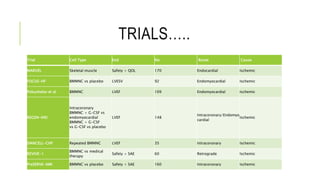
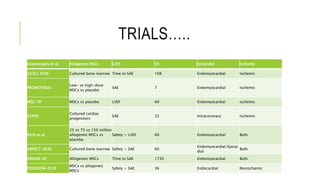
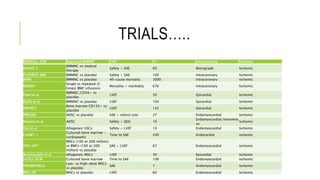
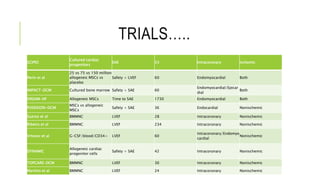
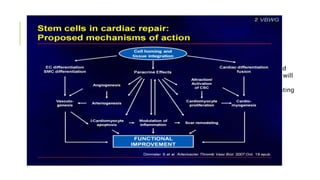
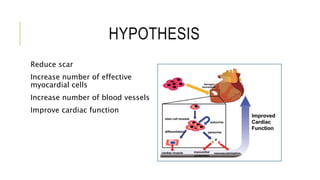
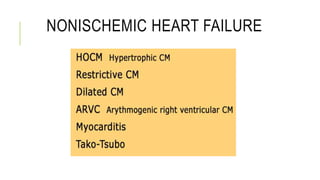
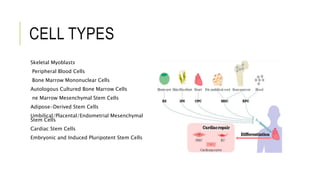
This document summarizes stem cell transplantation for heart failure, discussing the types of stem cells tested, delivery methods, and clinical trials. It describes how bone marrow mononuclear cells, mesenchymal stem cells, and cardiac progenitor cells have shown potential benefits in reducing scarring and improving cardiac function in preclinical and early clinical studies of ischemic and nonischemic heart failure. The most common delivery methods have been intramyocardial via endocardial or epicardial approaches and intracoronary infusion, with endomyocardial delivery being the most widely used technique clinically. Larger clinical trials are still needed to determine which cell types and delivery methods are most effective for treating heart failure.






![BONE MARROW MESENCHYMAL STEM
CELLS
Potential
Easily harvested and cultured
Immune-modulating and anti-inflammatory properties, making them immune-privileged.
MSCs typically express CD105, CD73, and CD90, but lack hematopoietic markers (CD45,
CD34, and CD14/CD11b)
Preclinical studies in ischemic and nonischemic cardiomyopathy
Angiogenesis, reduced fibrosis, and improved cardiac function
Clinical study in ischemic cardiomyopathy: improved patient functional capacity, quality
of life, and ventricular remodelling
Better than BMMNCs
Potential to be used as an allogeneic cell source, which leads to a very stable and reliable
cell source
Endocardial delivery of allogeneic bone marrow MSCs[DREAM-HF trial] in pipe line](https://image.slidesharecdn.com/stemcelltransplantationforheartfailure-150419010104-conversion-gate01/85/Stem-cell-transplantation-for-heart-failure-14-320.jpg)