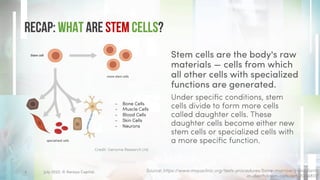
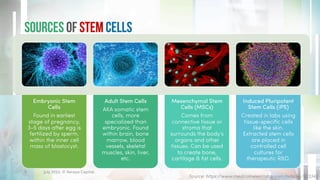
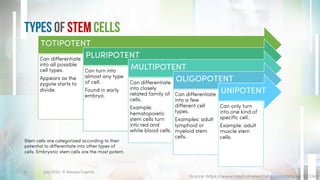
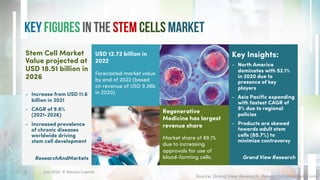
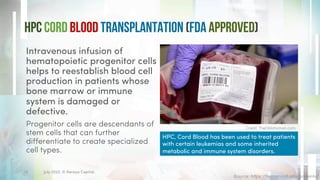
The document discusses recent developments in stem cell therapy, explaining its role as a non-invasive regenerative medicine that utilizes the body's natural healing capabilities to treat various conditions. It outlines the different types of stem cells, their sources, market projections, and potential applications, highlighting the necessity for new regulatory frameworks to support further advancements in this field. Despite promising results, less than a dozen stem cell therapies have been FDA-approved, emphasizing the need for continued research and development.