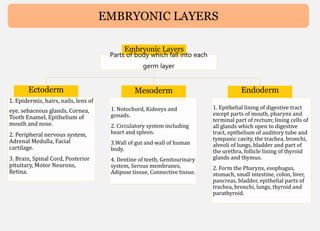
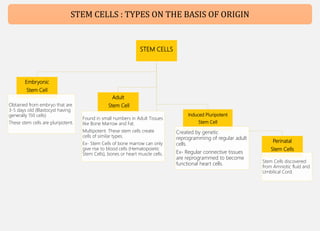
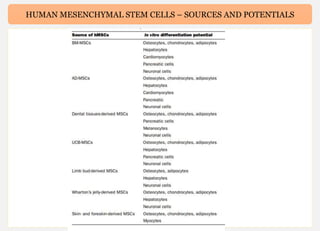
Human mesenchymal stem cells (hMSCs) are multipotent stem cells that can differentiate into cells of the mesoderm, ectoderm, and endoderm lineages. They are widely studied due to their self-renewal ability, multipotency, and low immunogenicity. hMSCs are found in various tissues including bone marrow, adipose tissue, and umbilical cord. They express specific cell surface markers and can differentiate into osteocytes, chondrocytes, and adipocytes. hMSCs show potential for use in regenerative medicine and cell therapy but face challenges such as maintaining potency during expansion. Their immunomodulatory properties and ability to home to sites of injury also make