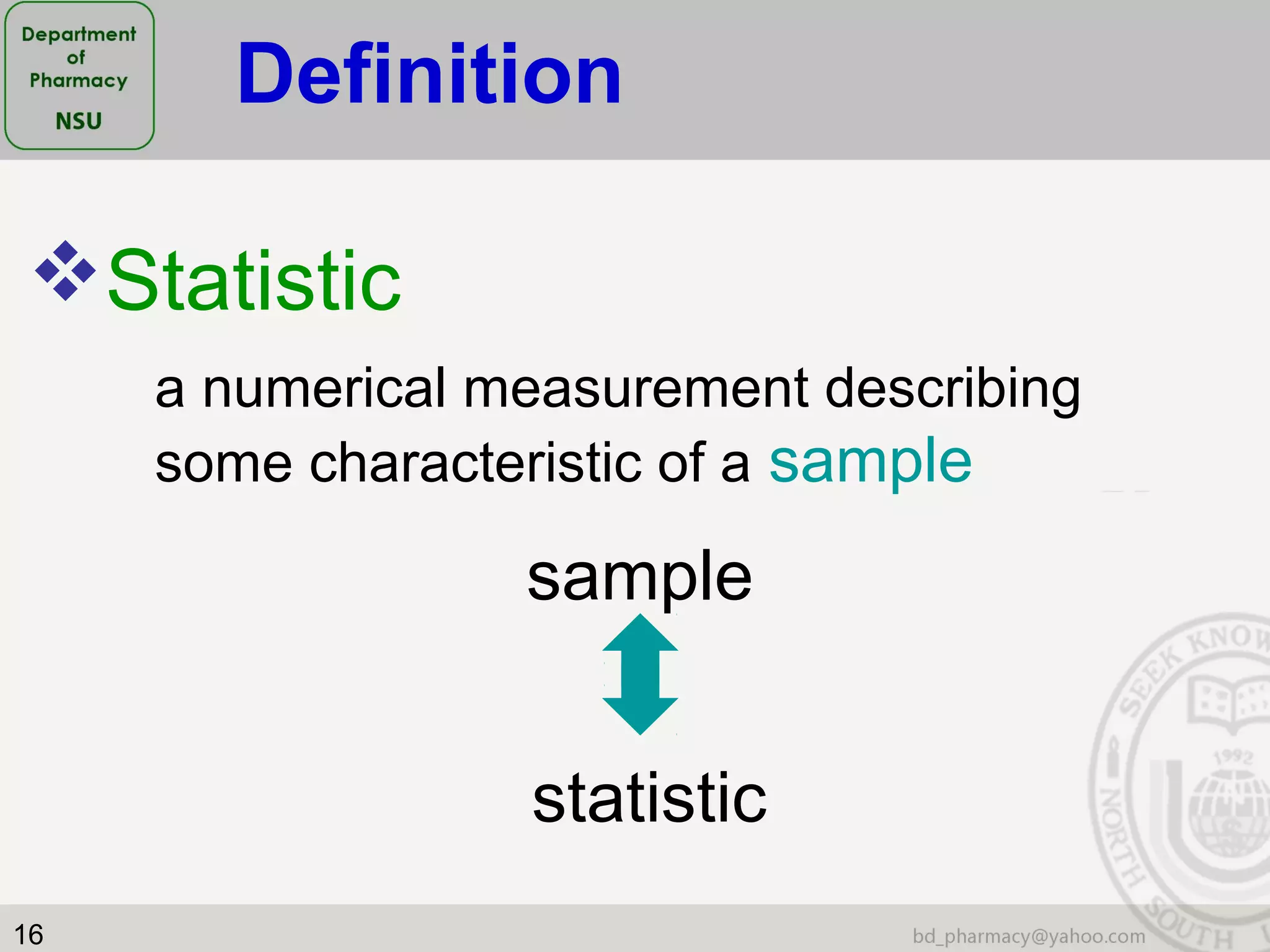
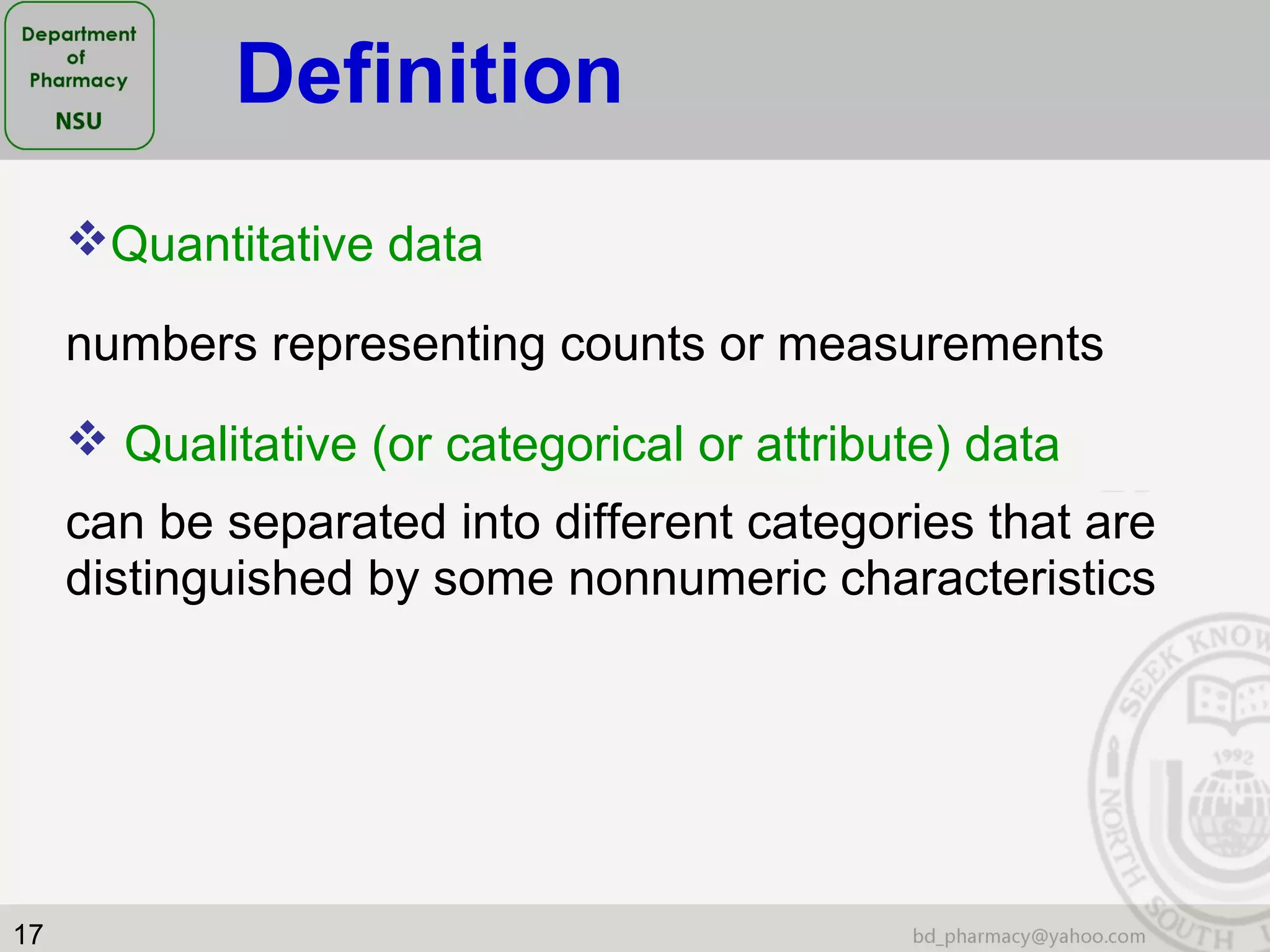
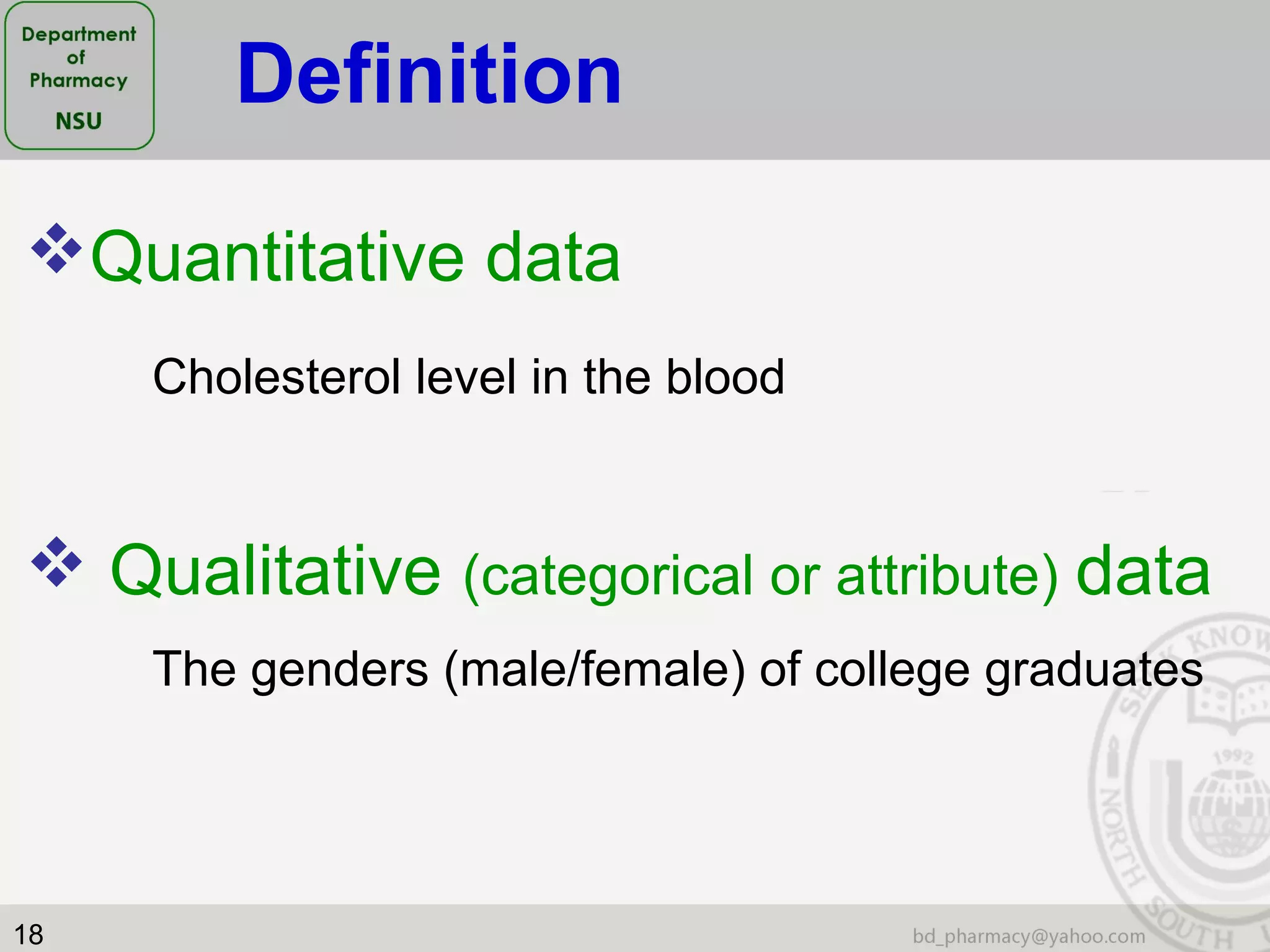
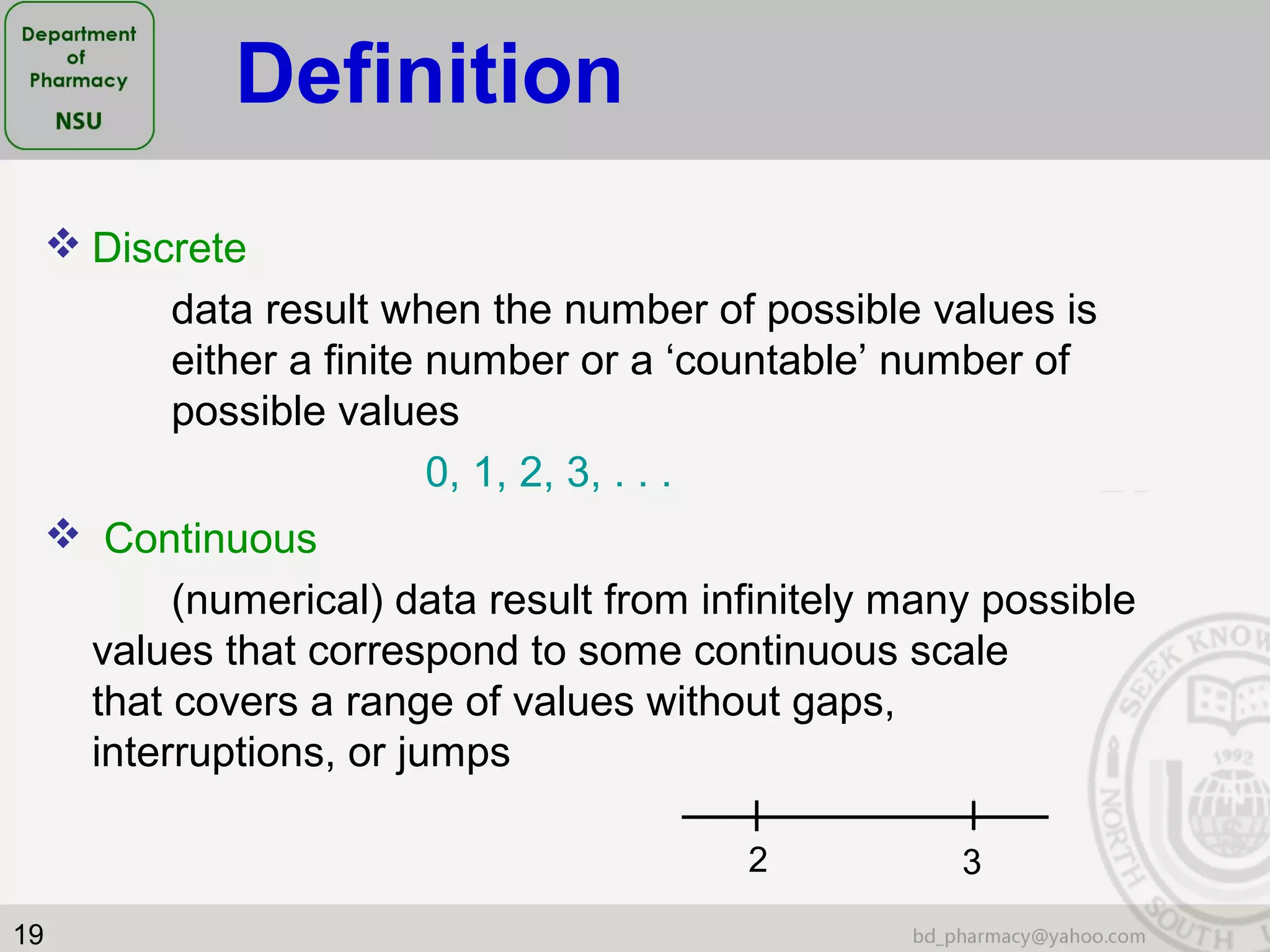
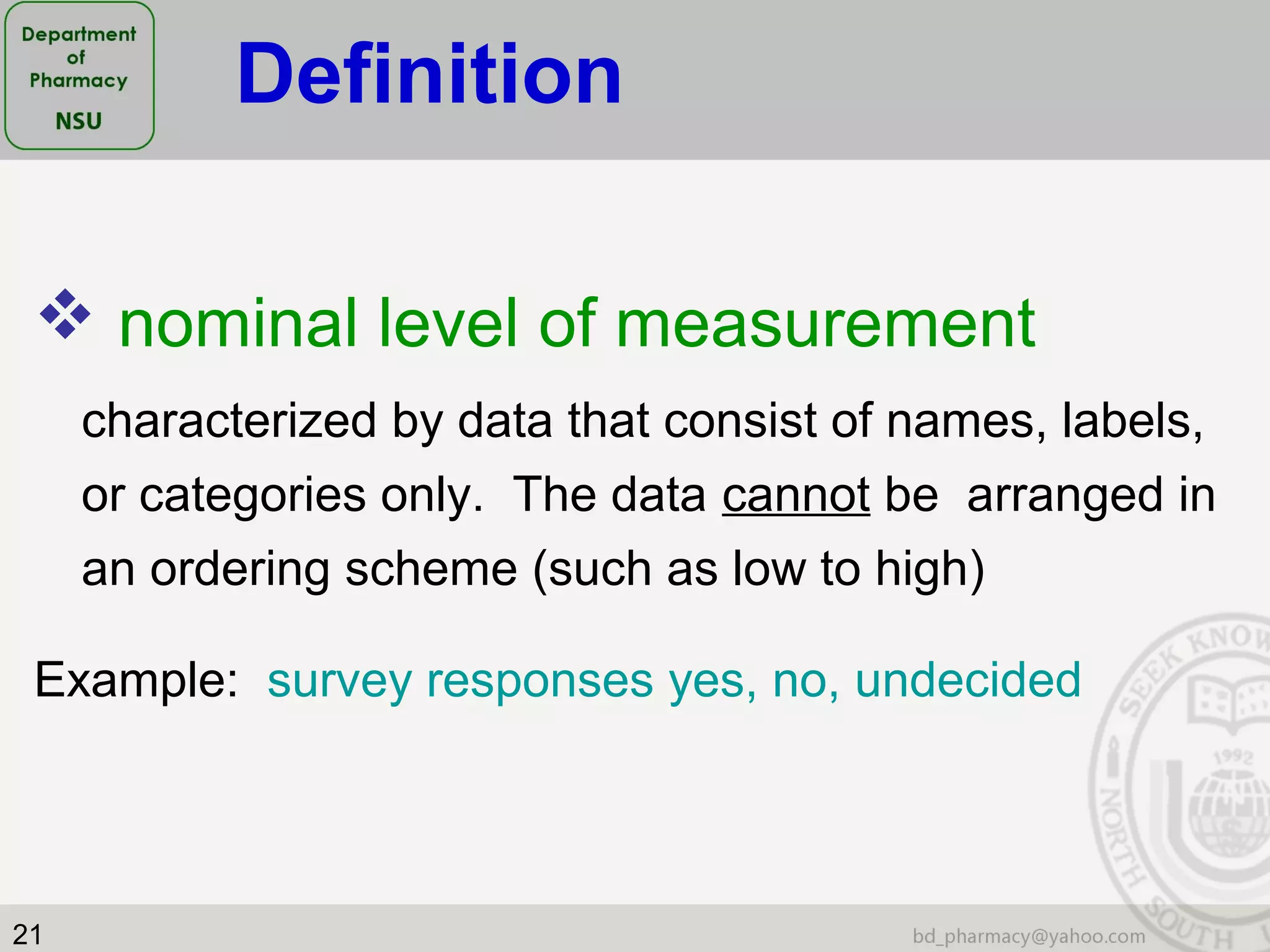
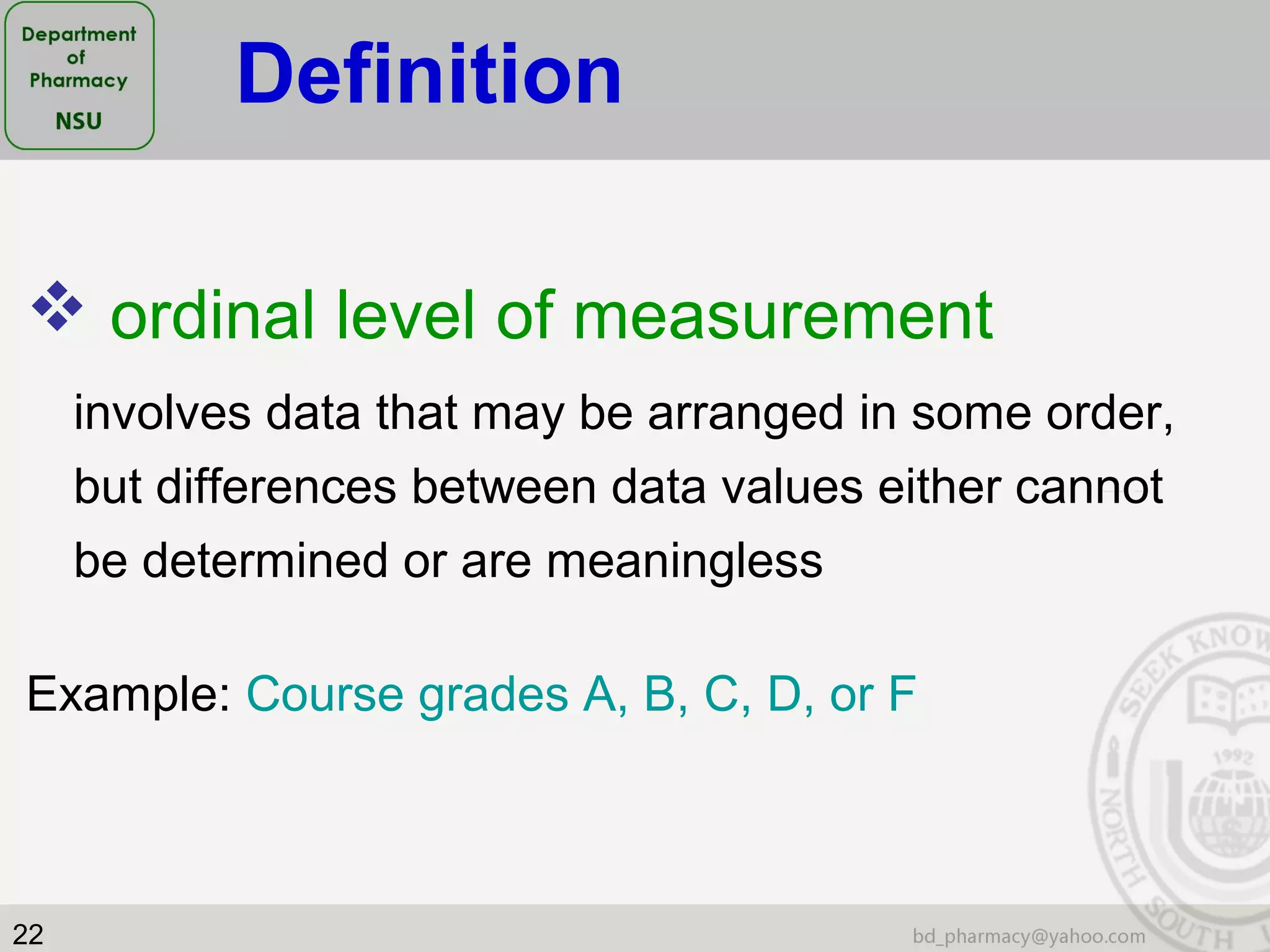
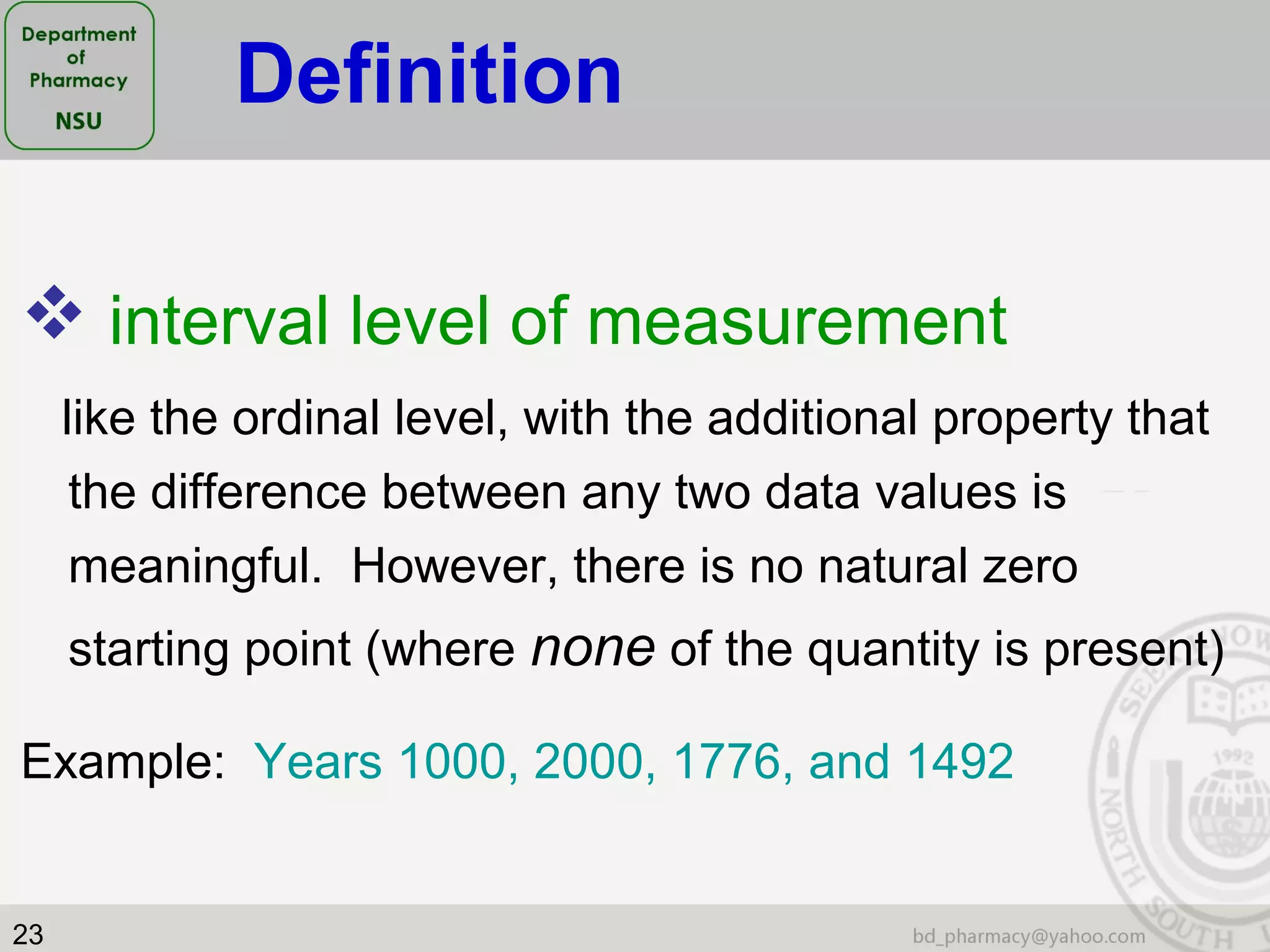
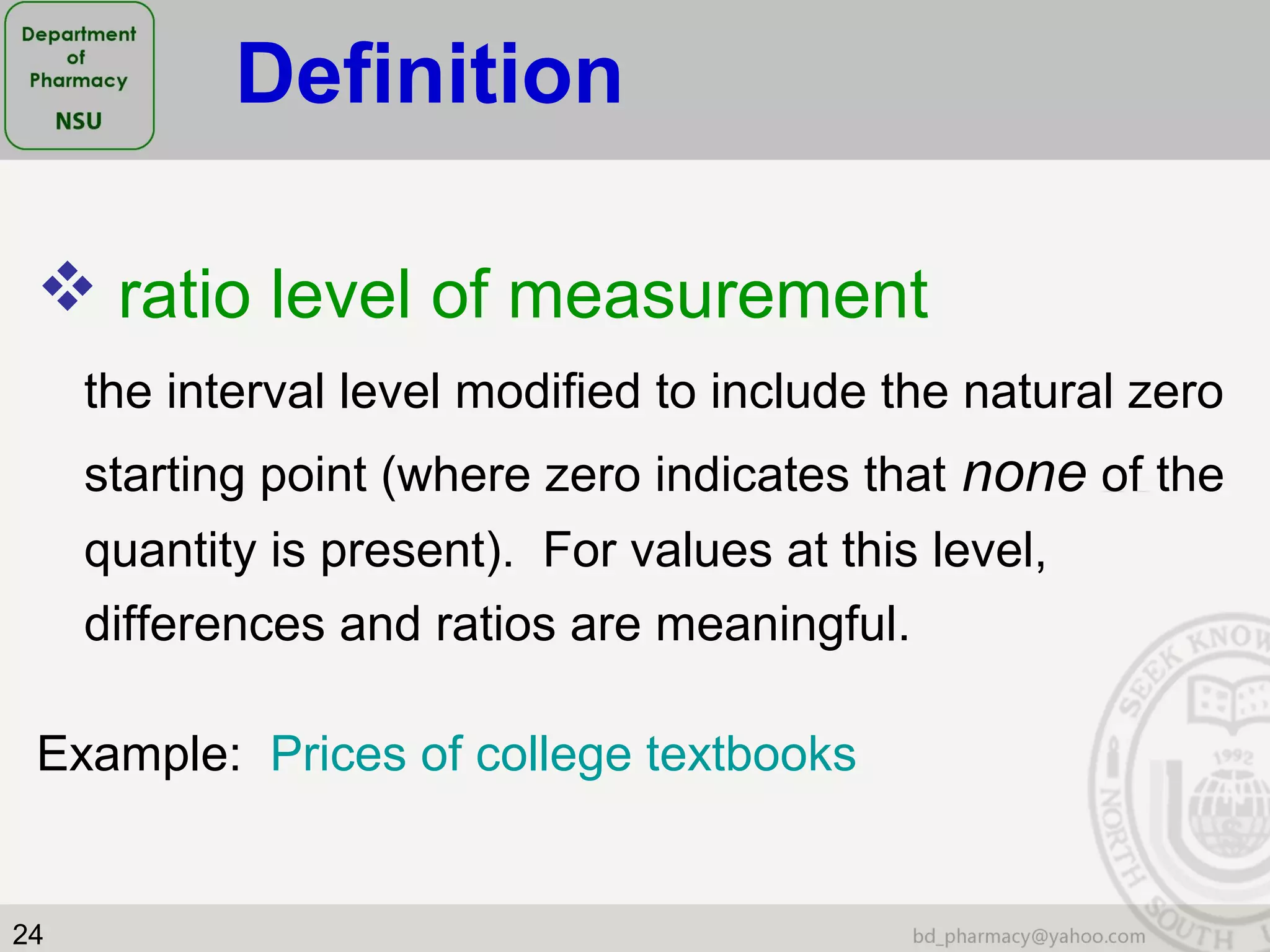
This document provides an overview of statistics concepts for a pharmacy course. It discusses topics like variables, populations and samples, levels of measurement for data, types of studies like randomized controlled trials, and key steps to planning a study. The document is intended to cover fundamental statistical concepts and their applications in pharmaceutical research and clinical trials.