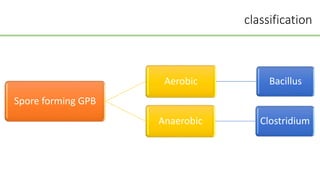
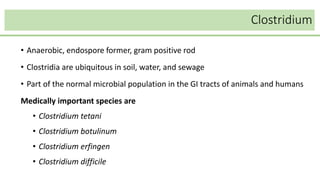
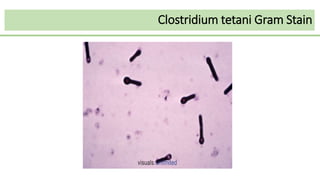
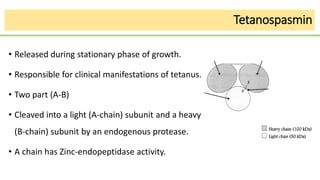
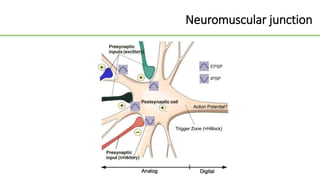
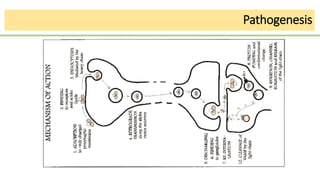
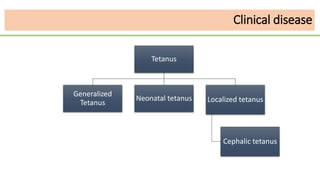
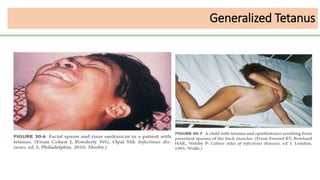
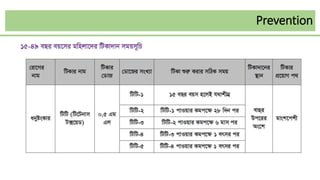
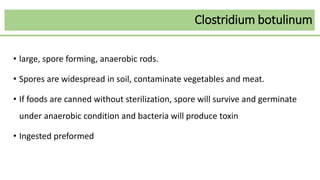
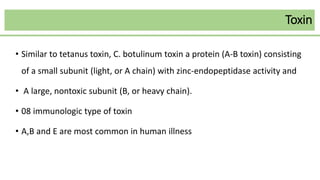
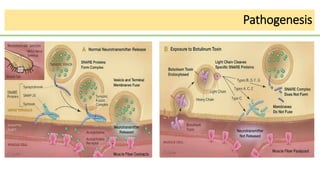
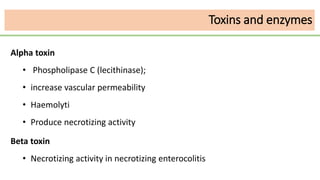
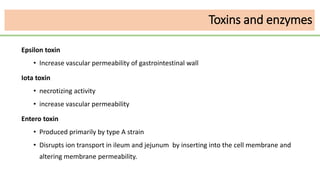
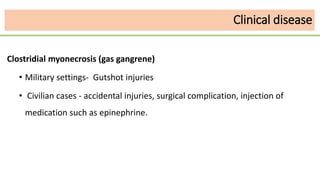
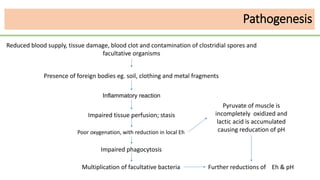
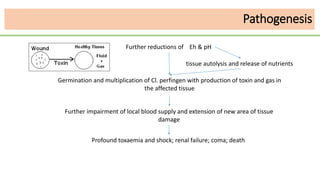
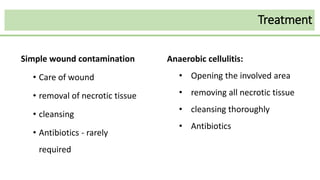
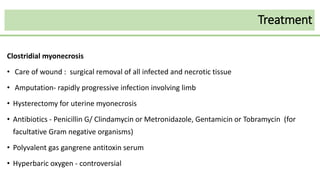
This document discusses several Clostridium species including C. tetani, C. botulinum, C. perfringens, and C. difficile. C. tetani causes tetanus and produces a neurotoxin that causes muscle spasms. C. botulinum produces a toxin that causes botulism resulting in paralysis. C. perfringens can cause gas gangrene through toxin production. C. difficile commonly causes antibiotic-associated diarrhea and pseudomembranous colitis in hospitals through toxin effects. These species are examined in terms of morphology, toxins, transmission, pathogenesis, diagnosis, and treatment.