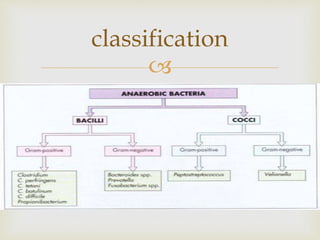
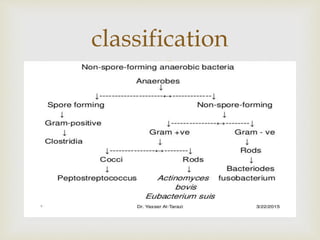
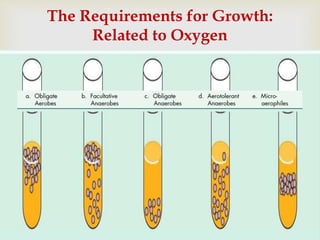
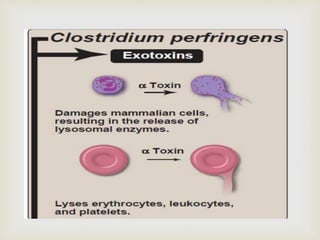
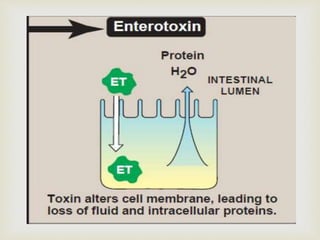
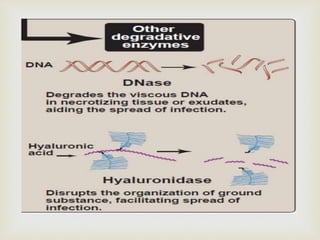
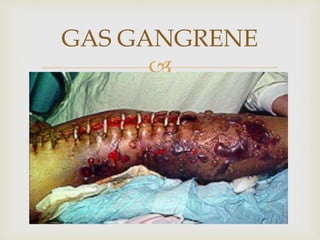
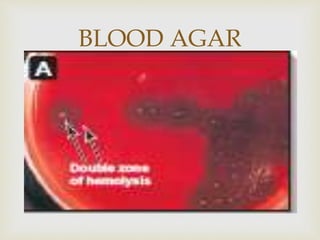
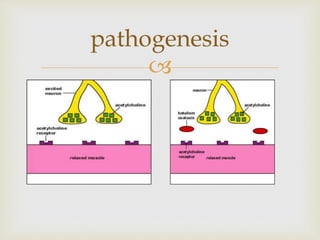
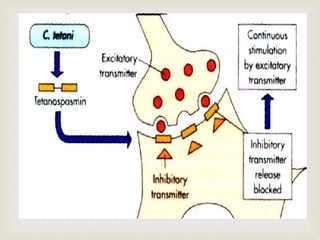
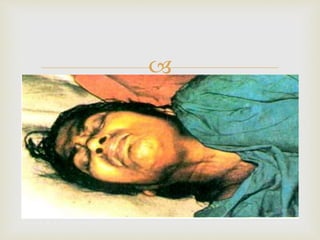
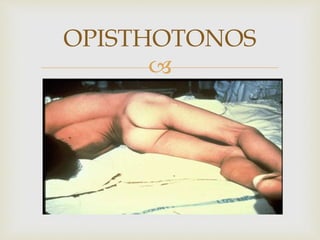
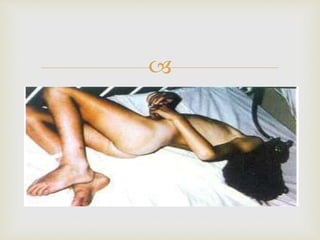
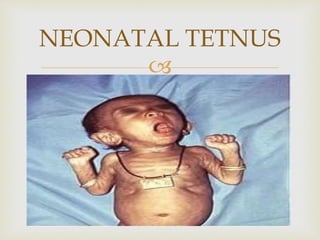
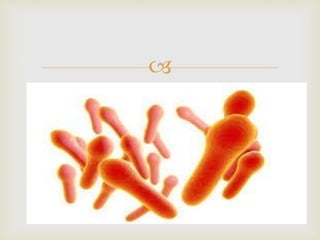
An anaerobe is an organism that does not require oxygen for growth. The document discusses various Clostridium species that are anaerobic bacteria. Clostridium perfringens can cause gas gangrene through secretion of exotoxins and enzymes. It is also a common cause of food poisoning. Clostridium botulinum produces a neurotoxin that causes botulism. Clostridium tetani secretes a toxin that causes the muscles spasms seen in tetanus. Clostridium difficile is associated with antibiotic-associated diarrhea and pseudomembranous colitis.