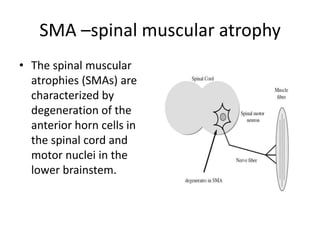
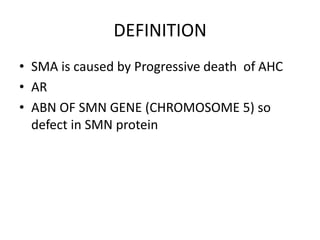
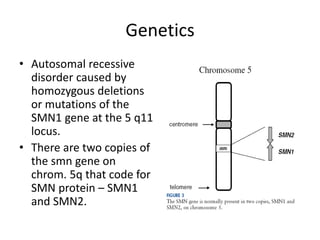
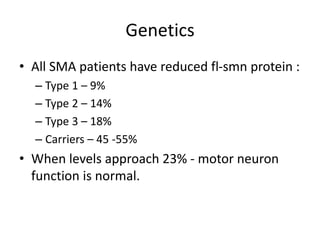
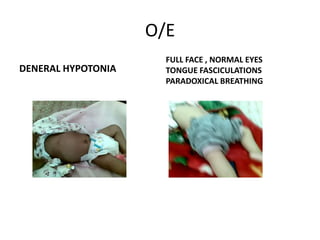
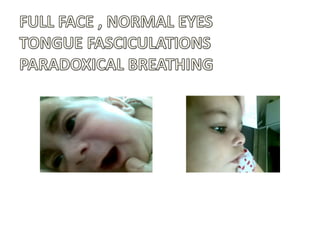
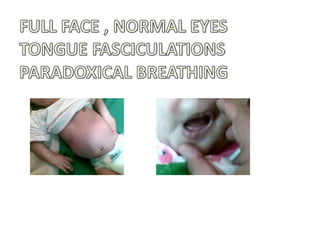
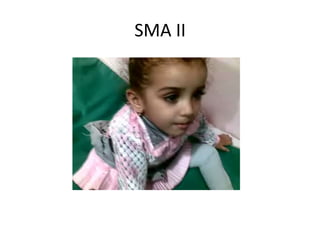
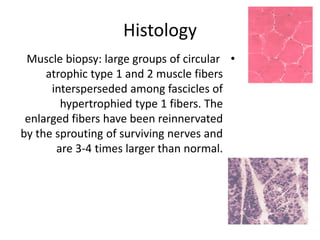
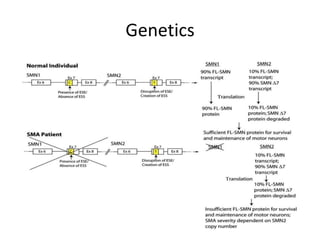
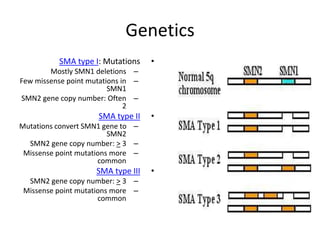
1. Spinal muscular atrophy (SMA) is caused by degeneration of motor neurons in the spinal cord and brainstem due to a defect in the SMN1 gene resulting in low levels of the SMN protein.
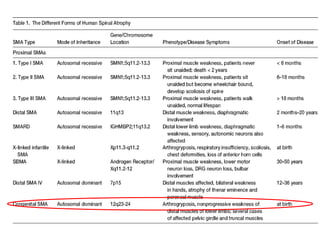
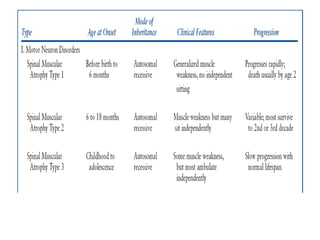
2. SMA is classified into five types based on age of onset and severity of symptoms - from very severe Type 1 to milder adult-onset Type 4.
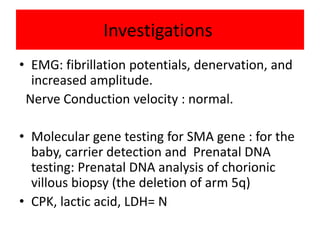
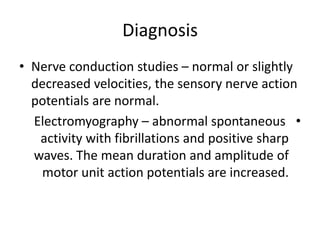
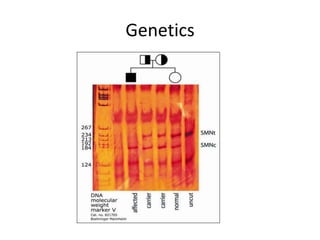
3. Diagnosis involves family history, physical exam, genetic testing, and other tests like EMG; there is currently no cure but supportive care can help manage symptoms.