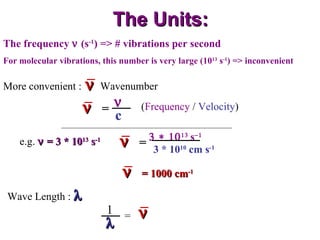
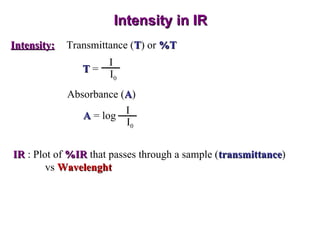
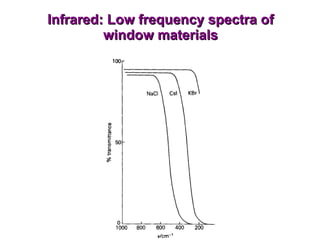
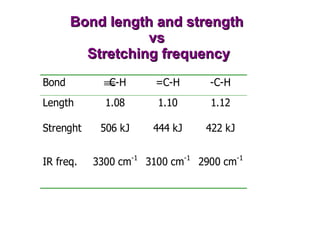
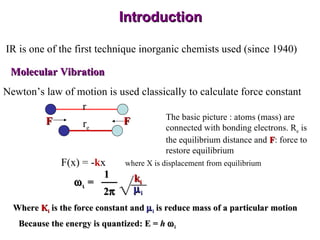
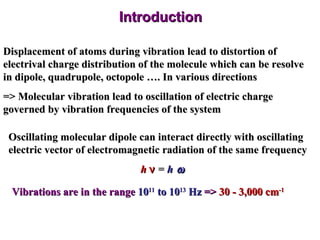
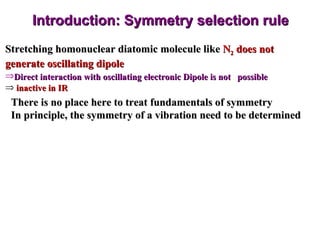
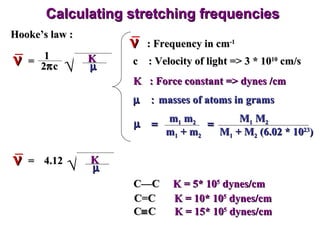
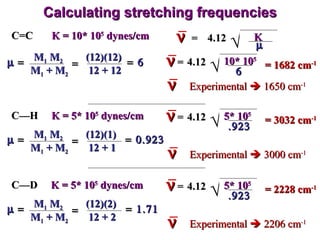
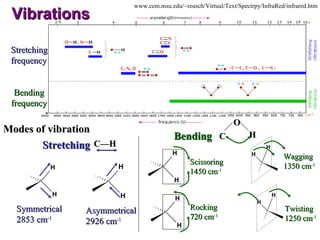
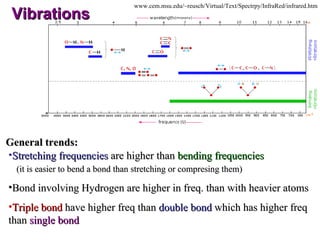
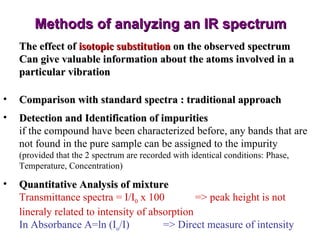
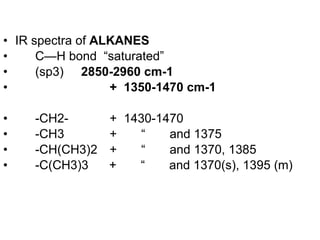
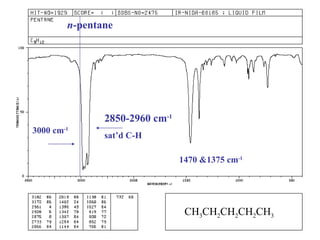
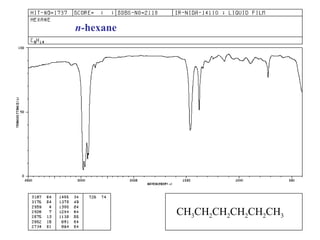
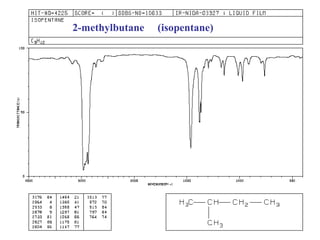
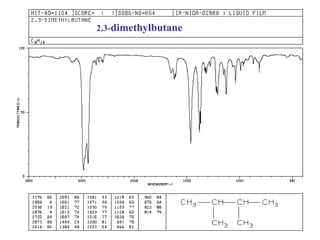
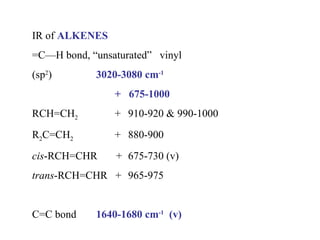
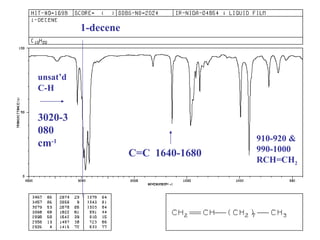
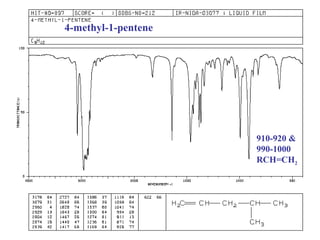
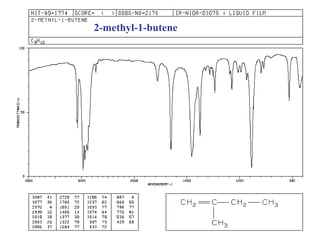
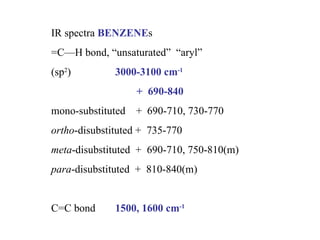
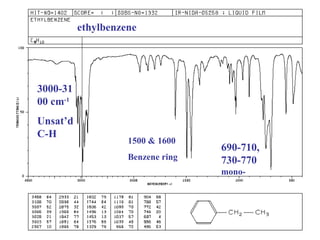
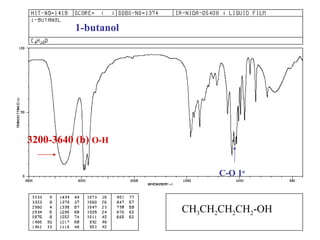
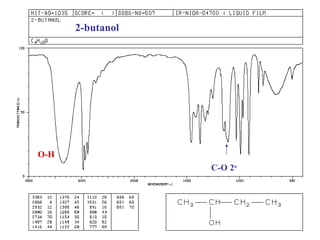
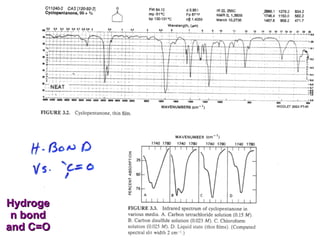
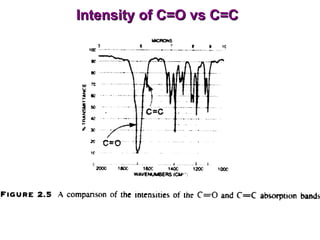
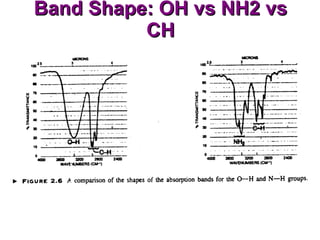
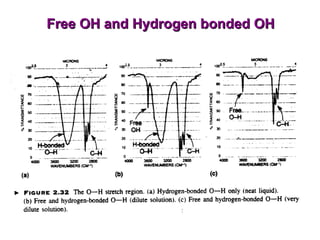
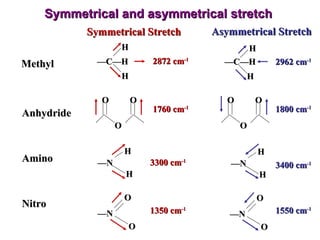
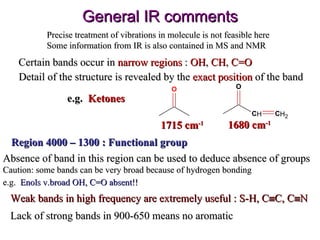
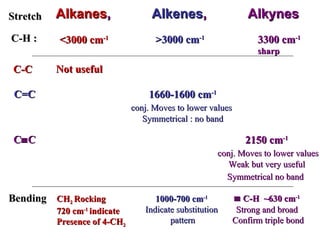
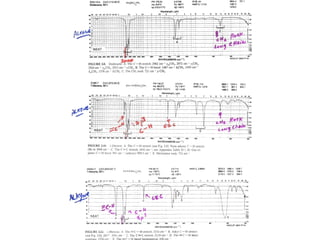
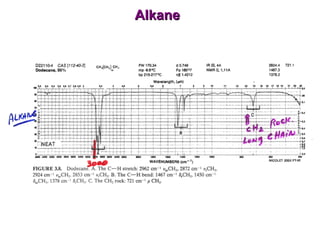
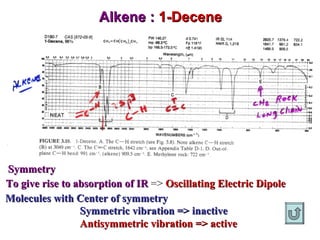
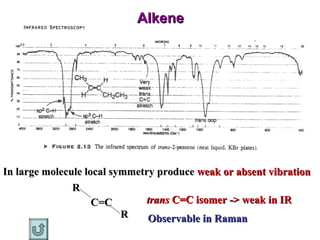
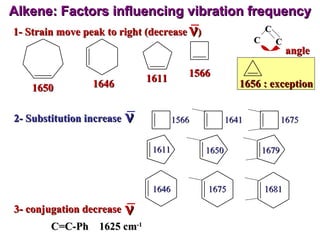
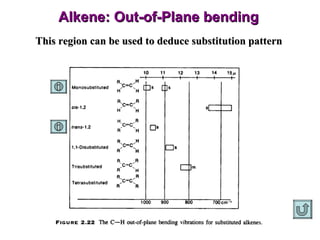
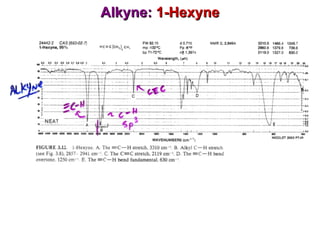
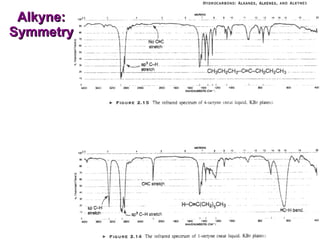
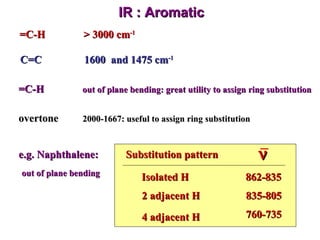
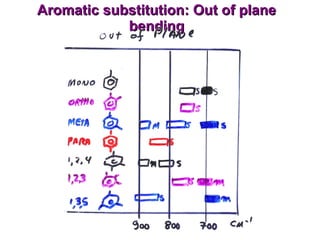
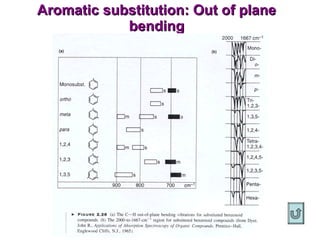
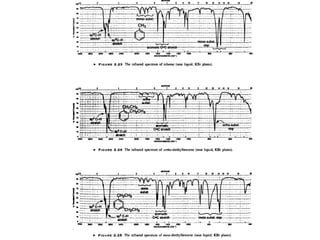
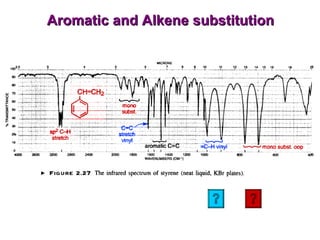
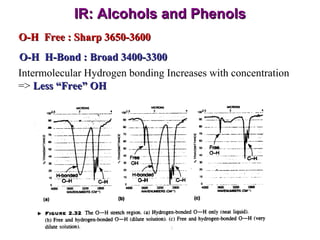
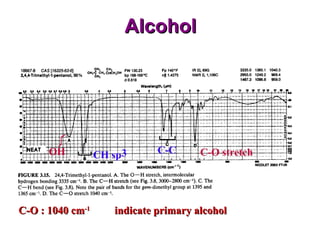
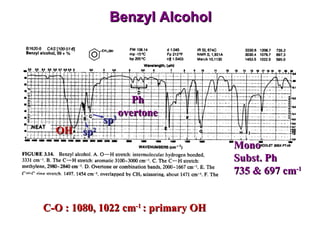
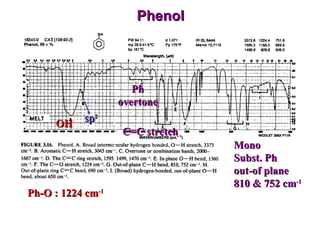
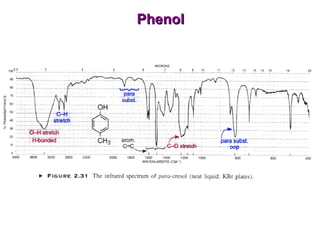
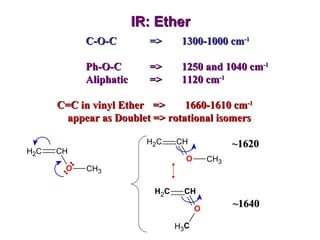
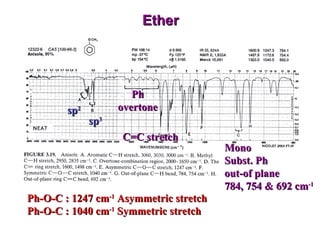
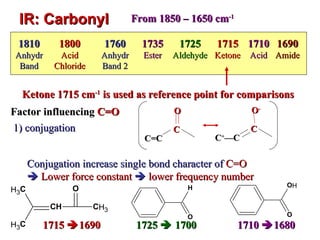
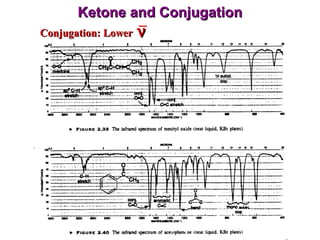
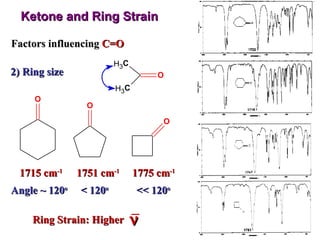
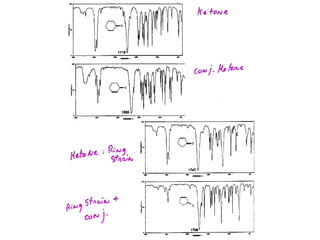
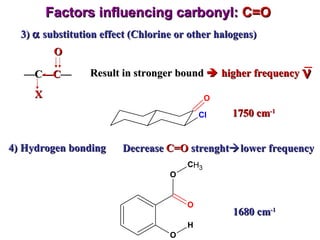
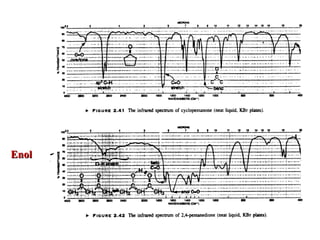
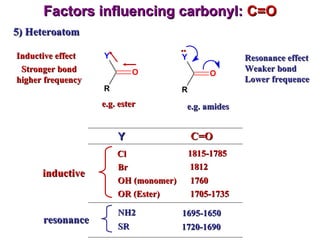
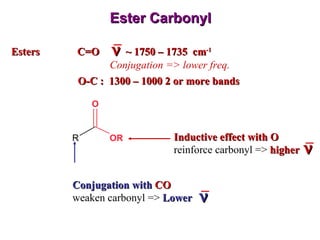
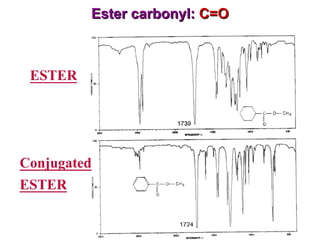
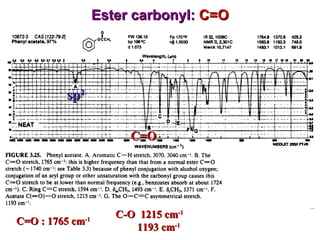
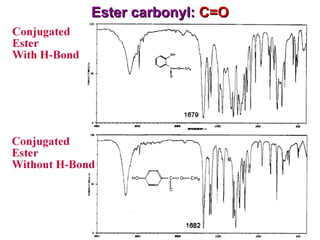
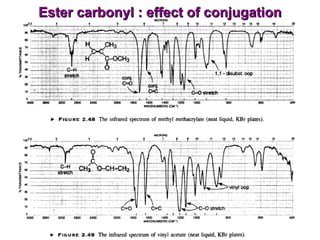
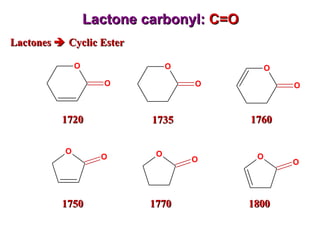
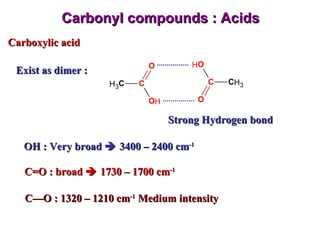
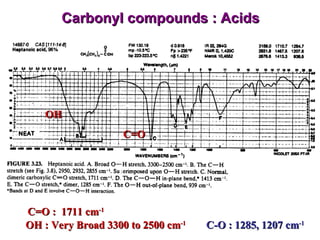
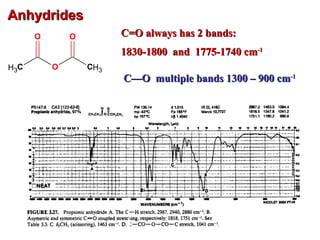
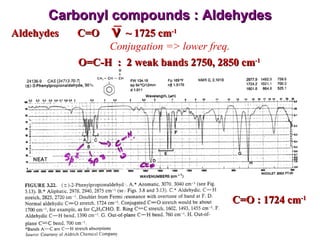
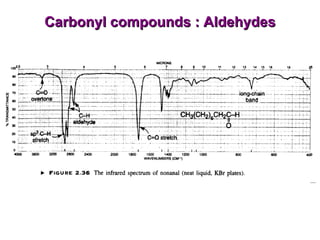
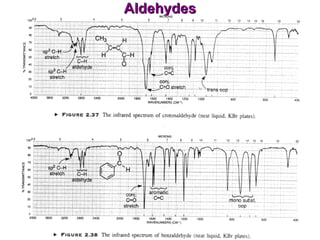
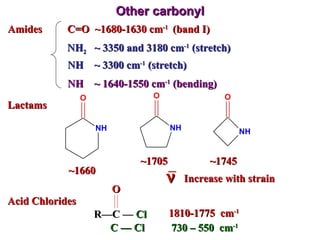
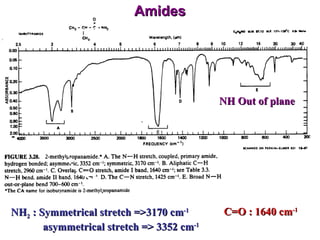
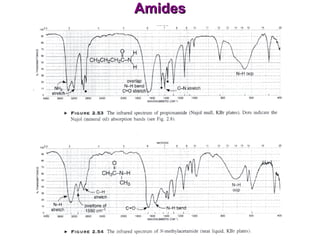
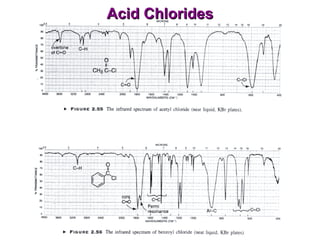
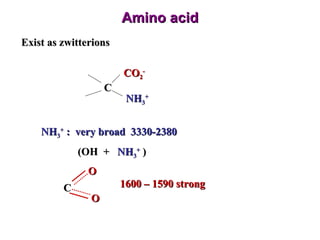
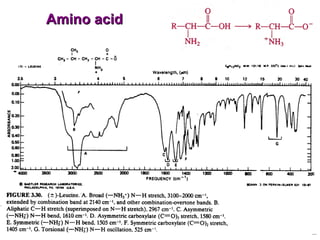
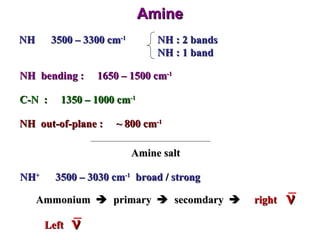
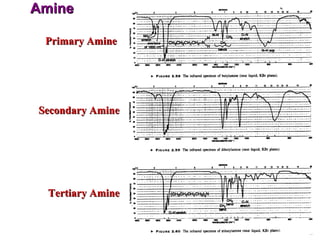
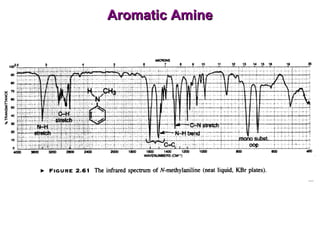
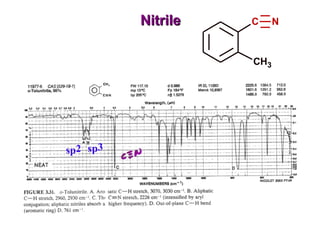
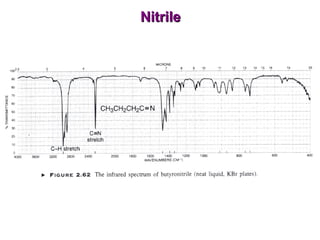
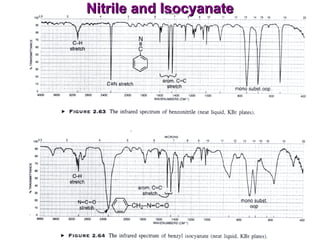
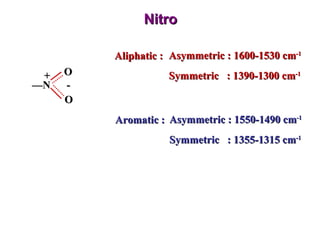
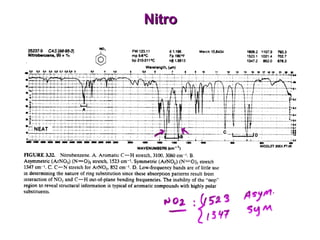
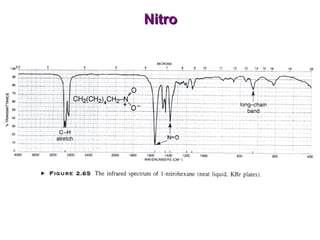
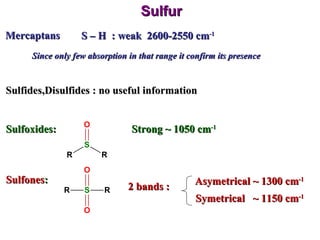
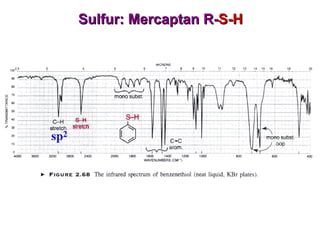
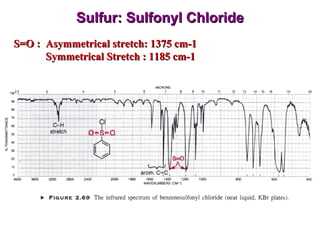
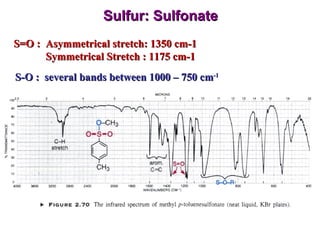
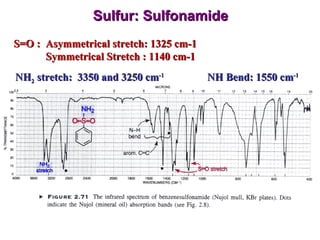
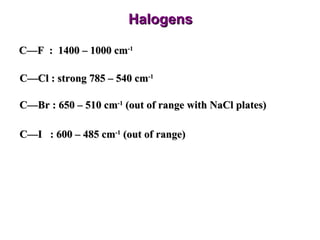
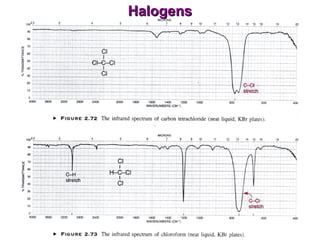
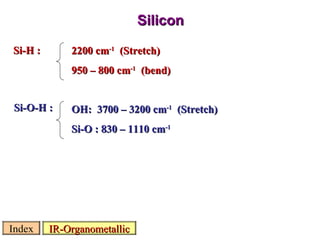
Infrared spectroscopy involves measuring the absorption or emission of electromagnetic radiation by molecules as they undergo transitions between different energy states. Infrared spectroscopy analyzes the infrared region of the electromagnetic spectrum, where molecules absorb radiation based on the vibrational and rotational motions of their bonds. The positions and intensities of absorption bands in an infrared spectrum provide information about the types of bonds in a molecule and can be used to determine its structure.