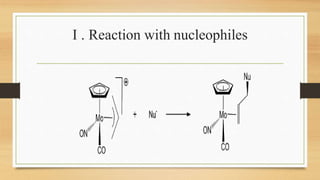
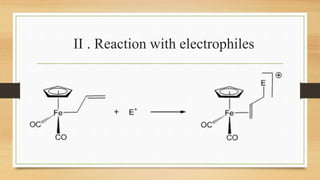
The document discusses metal allyl complexes, focusing on their bonding characteristics and molecular orbitals. It explains the different forms the allyl ligand can take (monohapto and trihapto) and their respective roles in coordination with transition metals. Additionally, it covers synthesis methods, reactions, and applications of these complexes, highlighting their significance in organometallic chemistry.