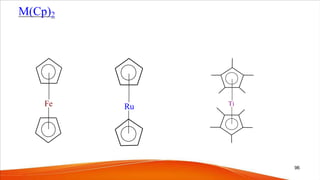
- Cyclopentadienyl (Cp) ligands are commonly found in organometallic complexes and form stable complexes with transition metals.
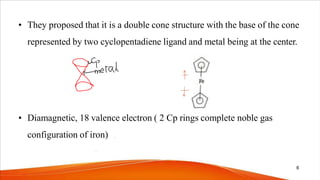
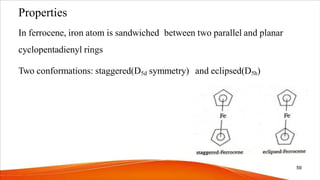
- Ferrocene was the first metallocene discovered accidentally in 1951 and has a "sandwich" structure with iron atoms in the center bonded between two parallel cyclopentadienyl rings.
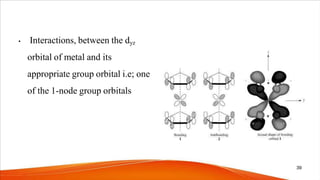
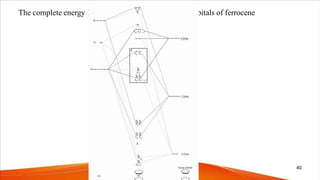
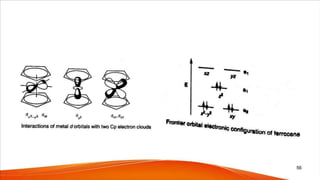
- The bonding in metallocenes like ferrocene involves interactions between the metal's d-orbitals and the π-orbitals of the cyclopentadienyl ligands, forming stable 18-electron complexes.




















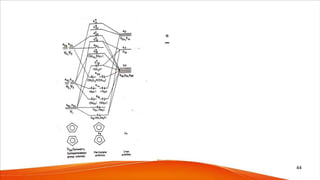







![Although isoelectronic with [Fe(Cp)2], [Co(Cp)2]+ (18e-)
shows more oxidative stability
=> In [Co(Cp)2]+ , Co is in Co(III), while in ferrocene Fe is
in Fe(II)
52](https://image.slidesharecdn.com/cyclopentadiene-tm2024-240409105415-dfe3740b/85/Cyclopentadiene-TM-Ferrocene-complex-pdf-52-320.jpg)

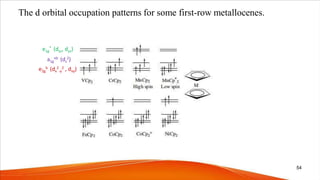
![[Mn(Cp)2] is high spin while [Re(Cp)2] is a low spin
complex
Re heavier congener of Mn
Hence higher crystal field splitting power
55](https://image.slidesharecdn.com/cyclopentadiene-tm2024-240409105415-dfe3740b/85/Cyclopentadiene-TM-Ferrocene-complex-pdf-55-320.jpg)

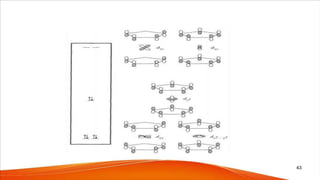
![• These three MOs can accommodate 6 electrons.
• In this event, the lower 6 MOs (i.e. upto e1u
b) remain more or less
unpurturbed.
• These 6 MOs accommodate 12 electrons and 3 new MOs can
accommodate 6 electrons to give the 18e configuration
• Ex: [(η5-Cp)2Re-H], [(η5-Cp)2Mo(H)2], [(η5-Cp)2Ti(CO)2], [(η5-
Cp)2Ta(H)3]
• For ex, Cp2Re(17e) and Cp2Re+ (16e) are unstable, Cp2ReH is very
stable
58](https://image.slidesharecdn.com/cyclopentadiene-tm2024-240409105415-dfe3740b/85/Cyclopentadiene-TM-Ferrocene-complex-pdf-58-320.jpg)



![• Cyclopentadienyl derivatives of
TMs available in various
ox.states
Ex: [CpMo(CO)3]- , CpMn(CO)3 ,
Fe(Cp)2 , [Co(Cp)2]+ , TiCl2(Cp)2 ,
NbBr3(Cp)2
• with central metals in
0,1,2,3,4 & 5 ox.states
respectively
• Metallocenes from the 3d
transition series - generally
paramagnetic
• Exception: Fe(η5-C5H5)2 ,
[Co(η5-C5H5)2]+ and Ti(η2-
Cp)2
63](https://image.slidesharecdn.com/cyclopentadiene-tm2024-240409105415-dfe3740b/85/Cyclopentadiene-TM-Ferrocene-complex-pdf-63-320.jpg)













![Central chirality
Central chirality also known as lateral
chirality is the second type of chirality
found in ferrocene and similar
compounds.
It is basically due to a chiral carbon
centre directly attached to the Cp ring.
Ex:Ugi’s amine, [(R)-N,N-dimethyl-1-
ferrocenylethylamine]
95](https://image.slidesharecdn.com/cyclopentadiene-tm2024-240409105415-dfe3740b/85/Cyclopentadiene-TM-Ferrocene-complex-pdf-95-320.jpg)