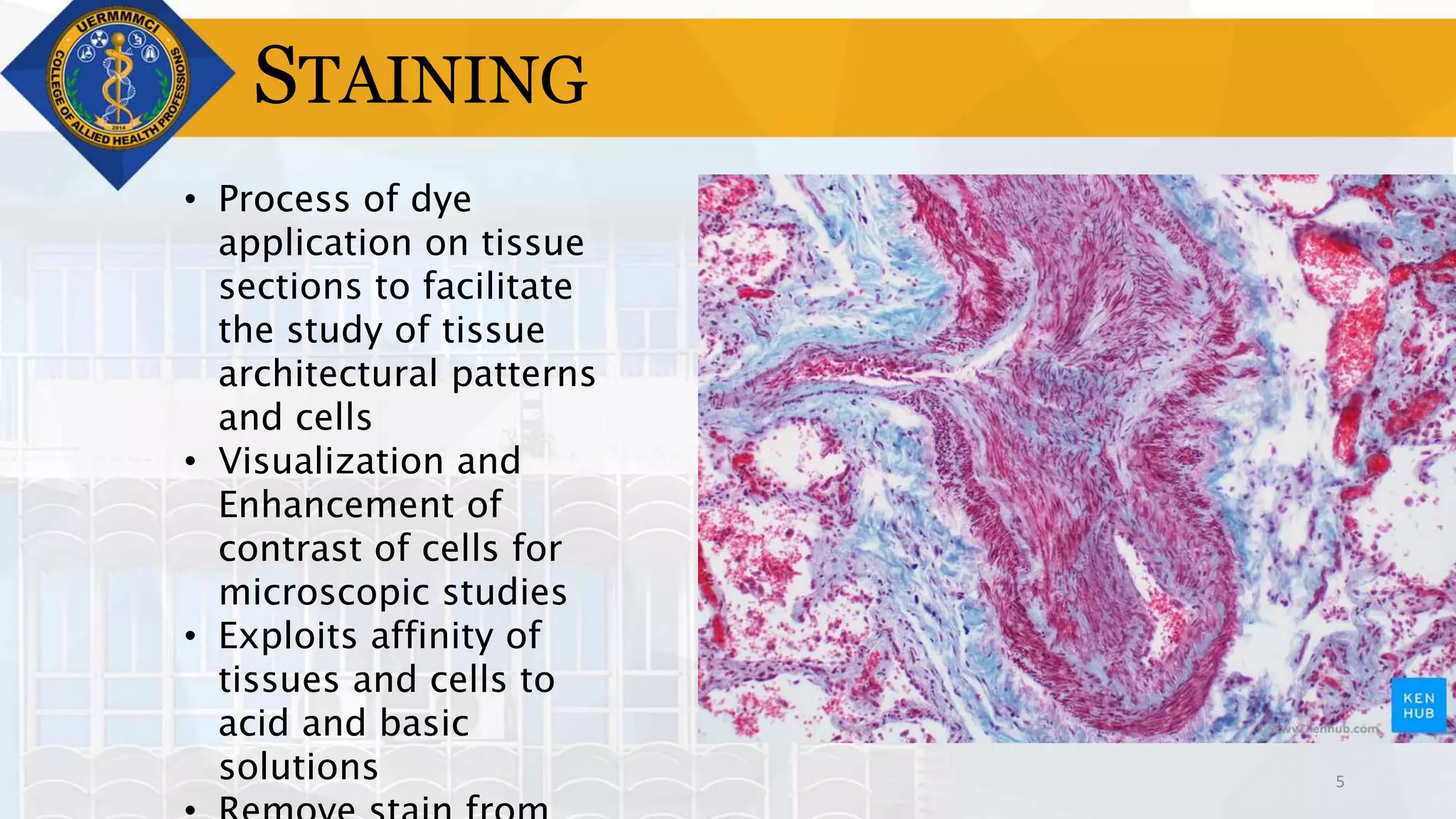
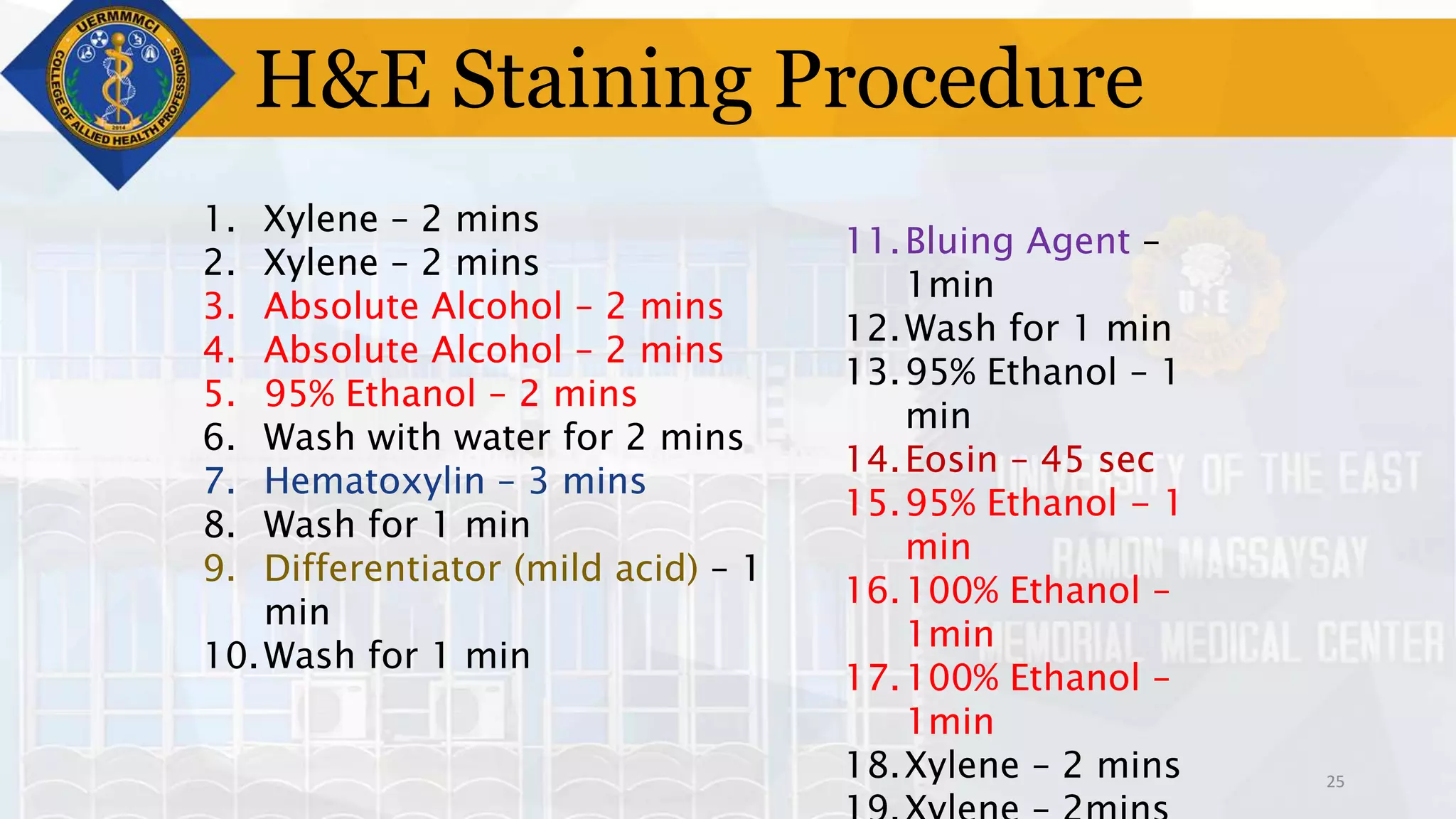
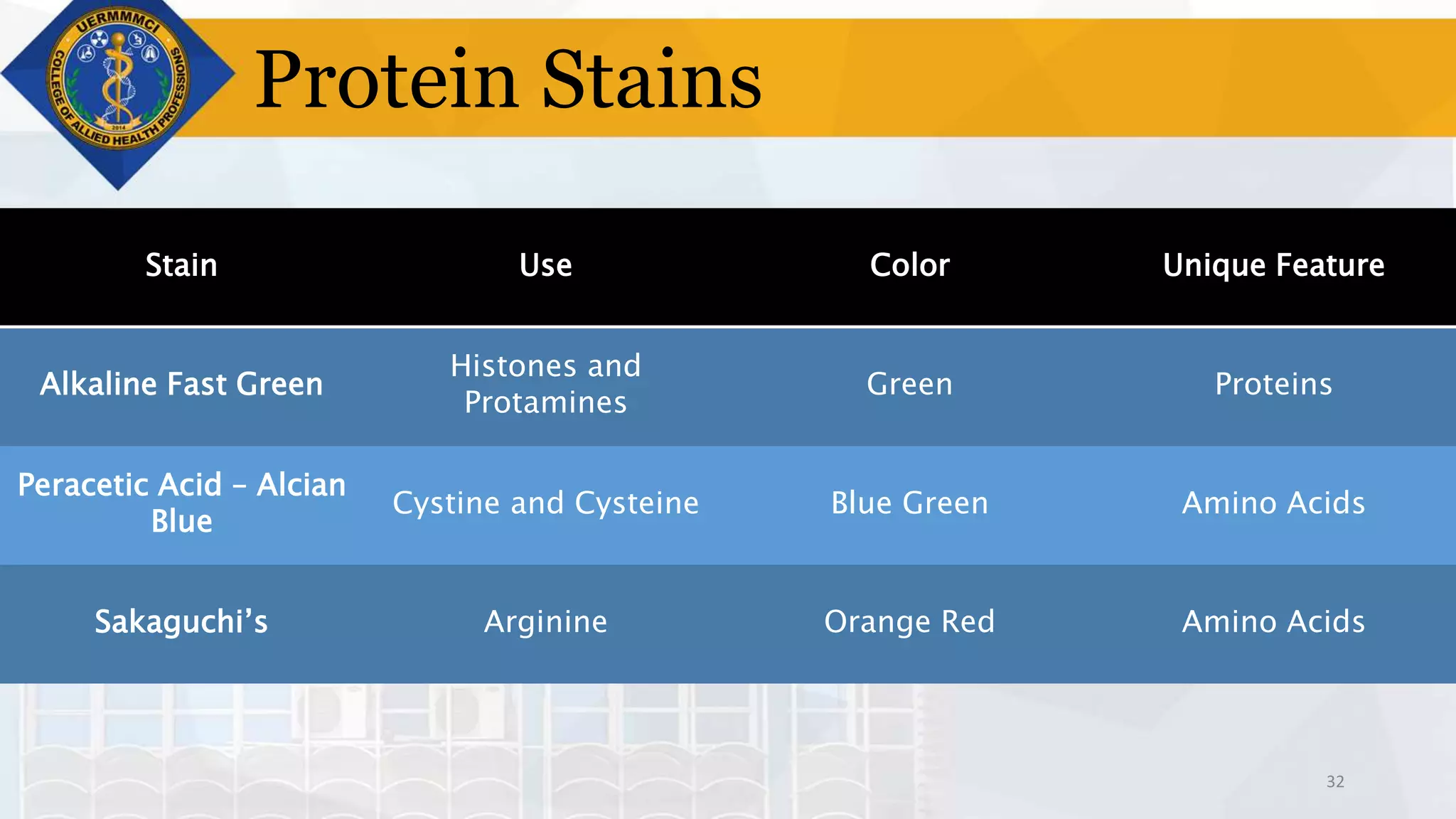
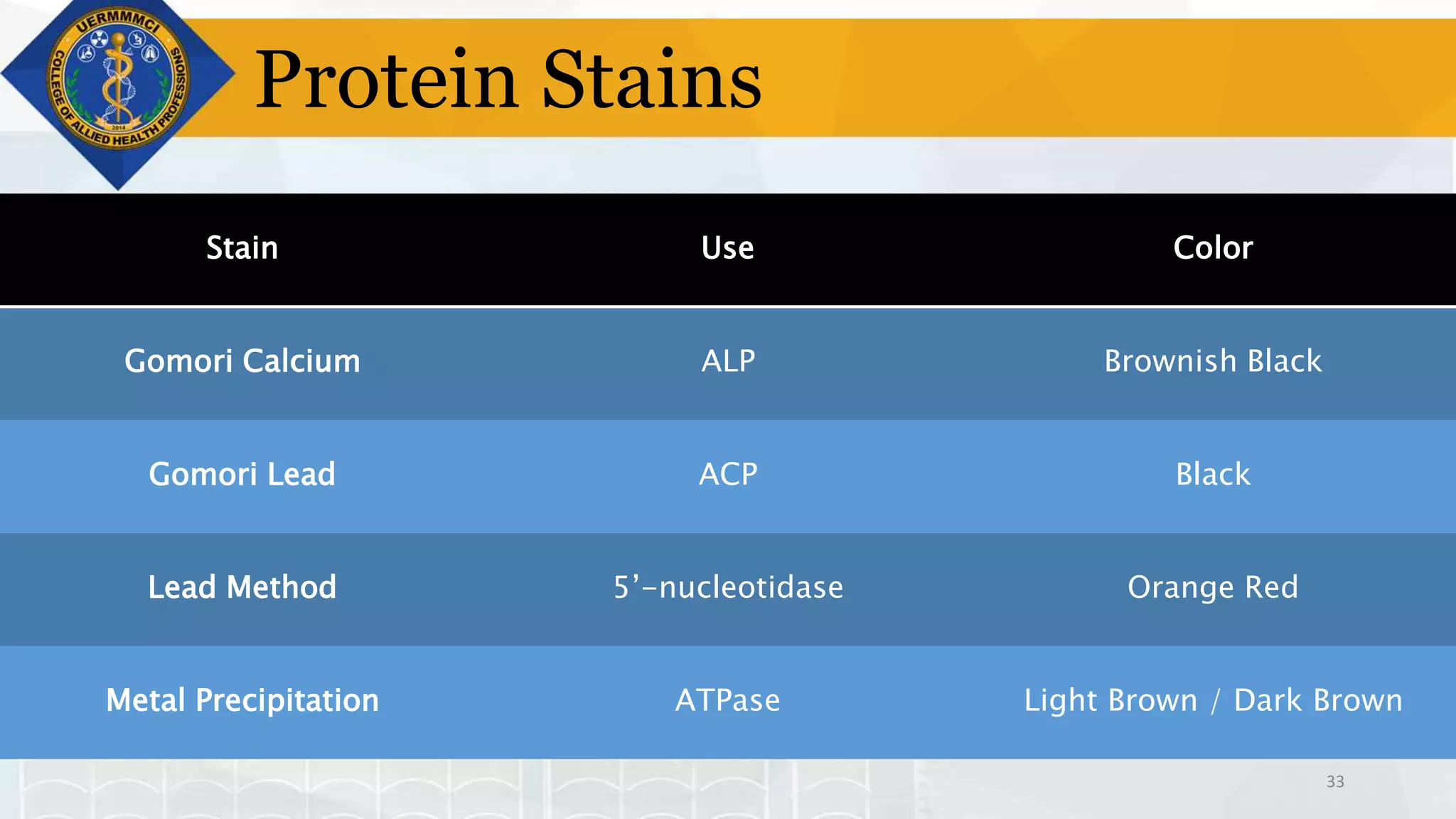
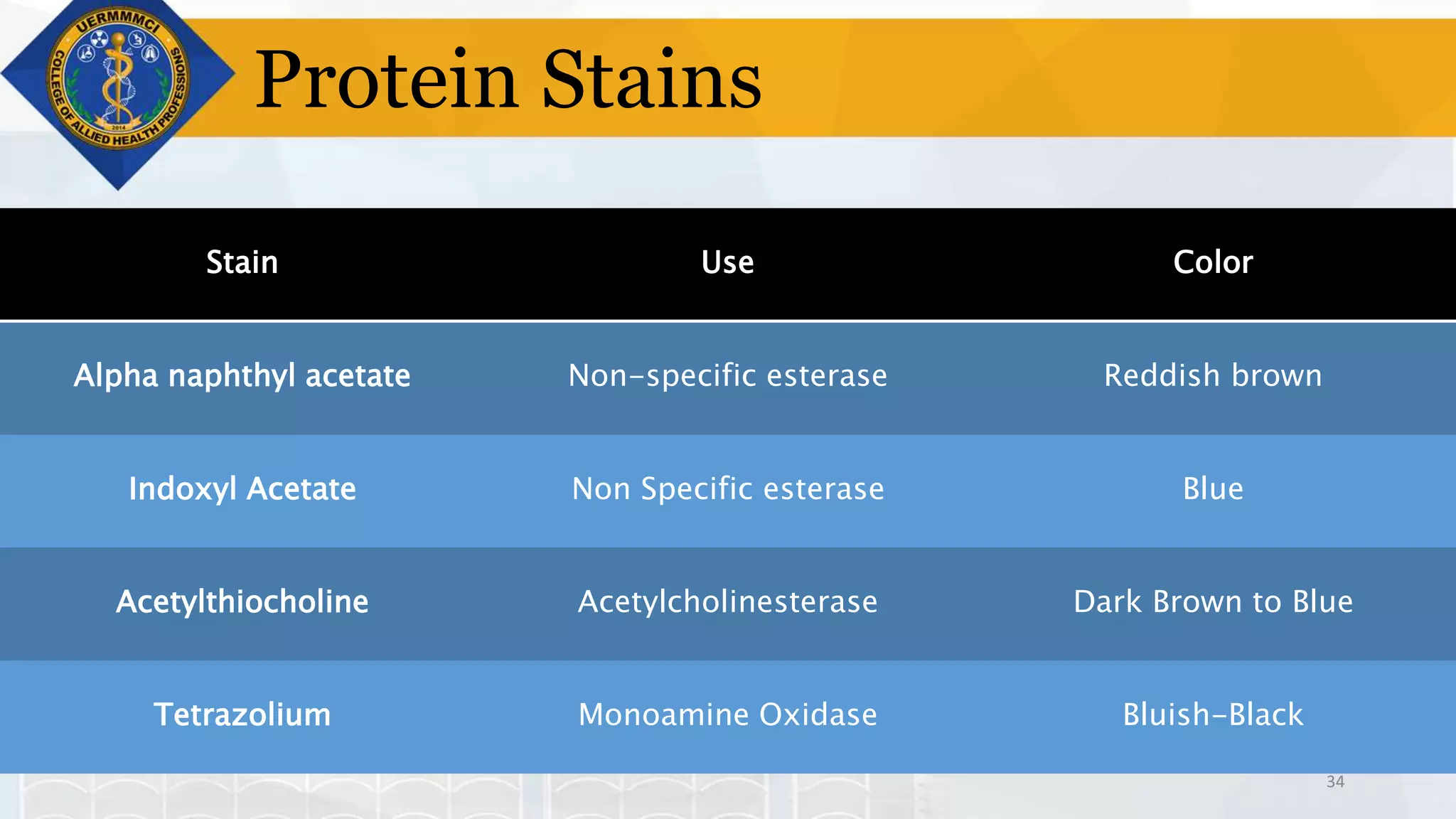
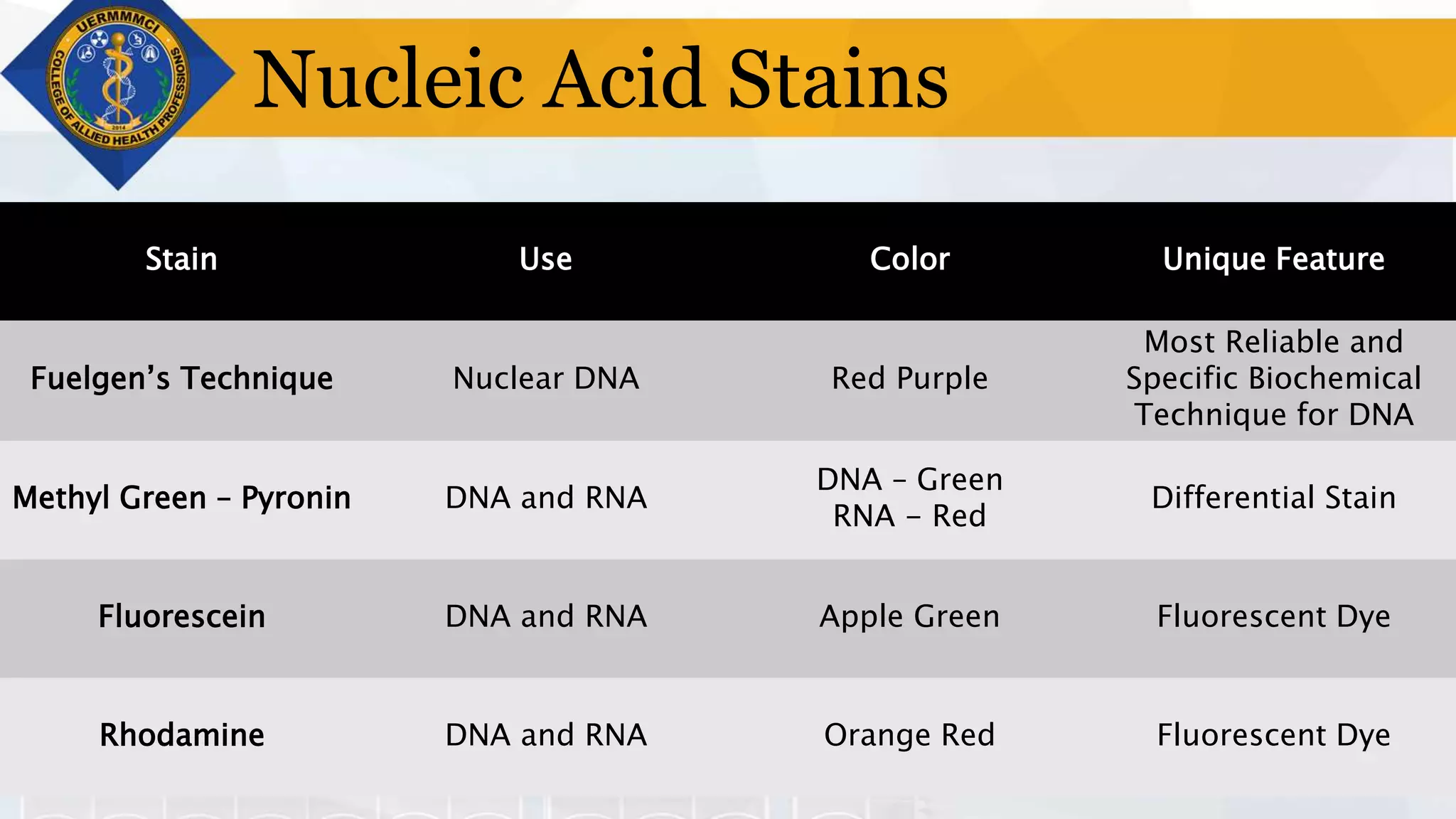
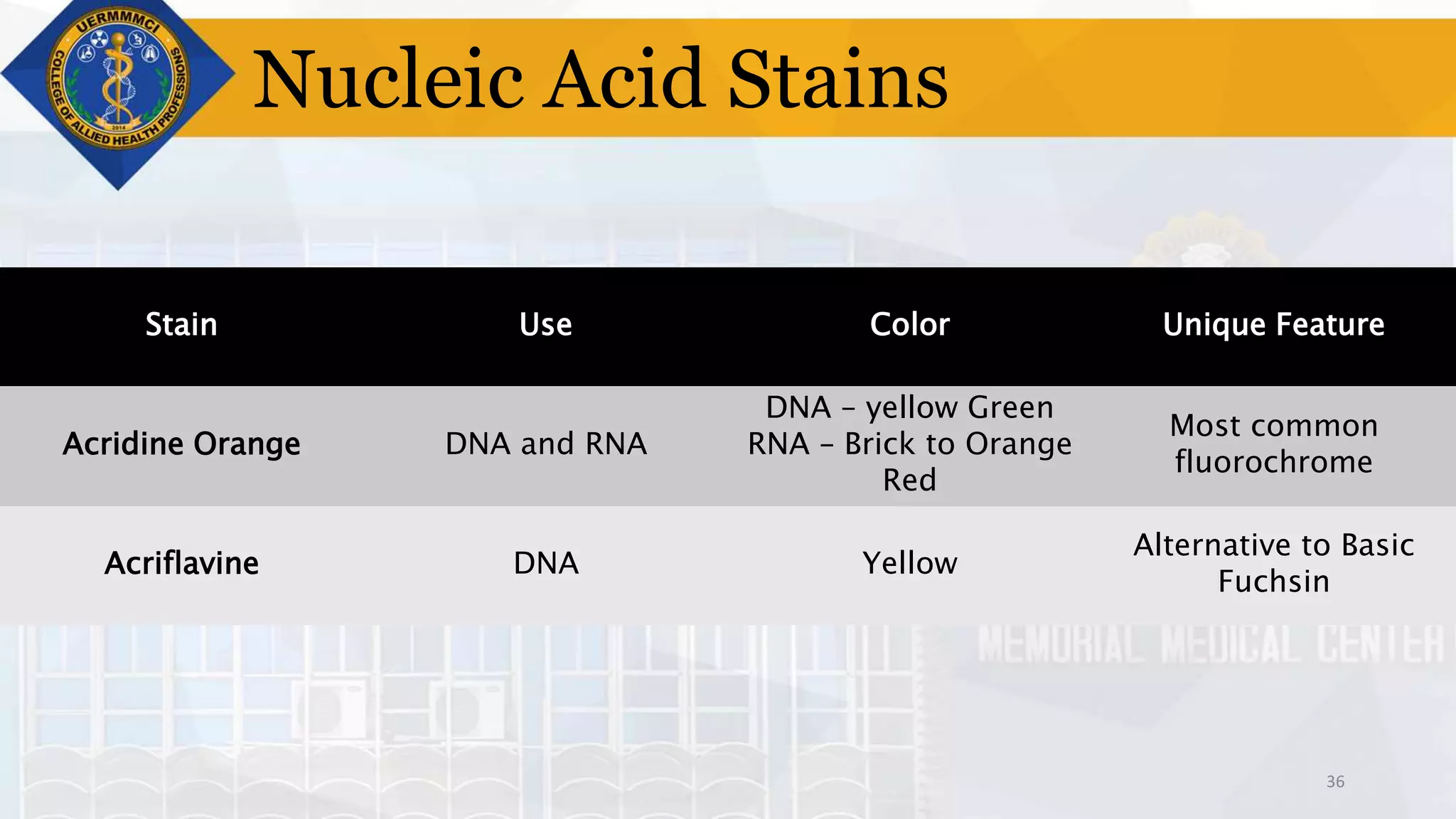
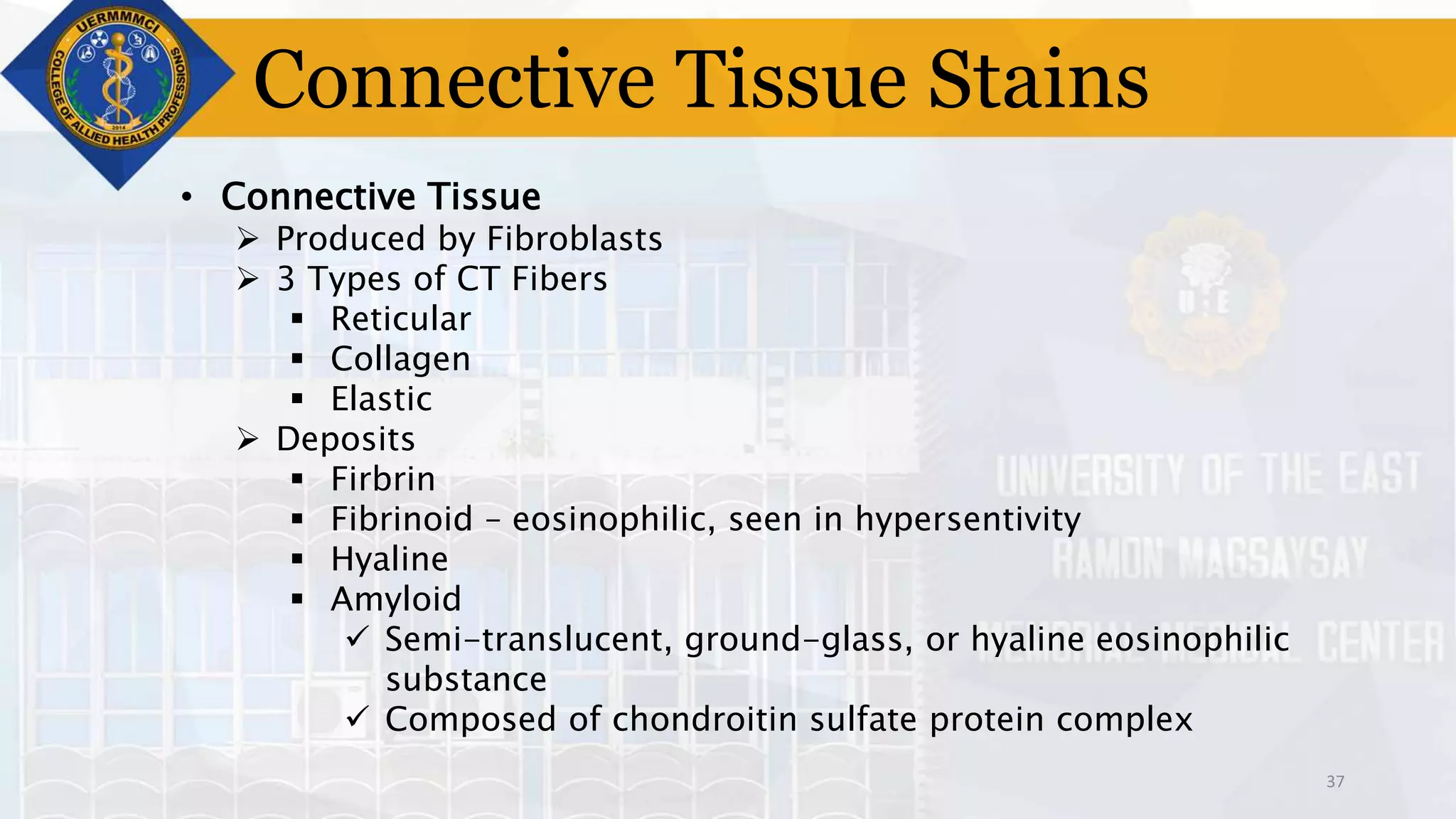
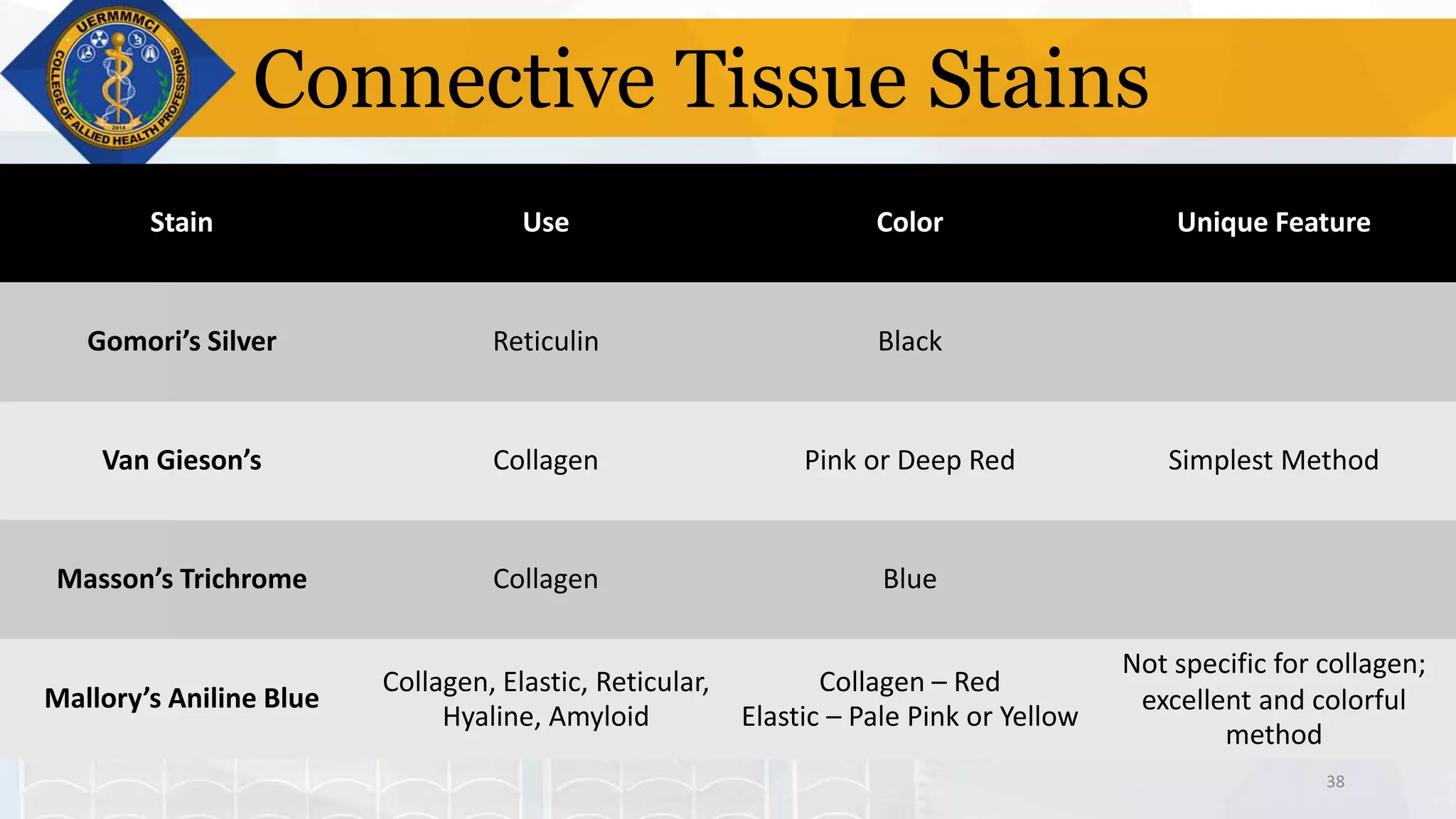
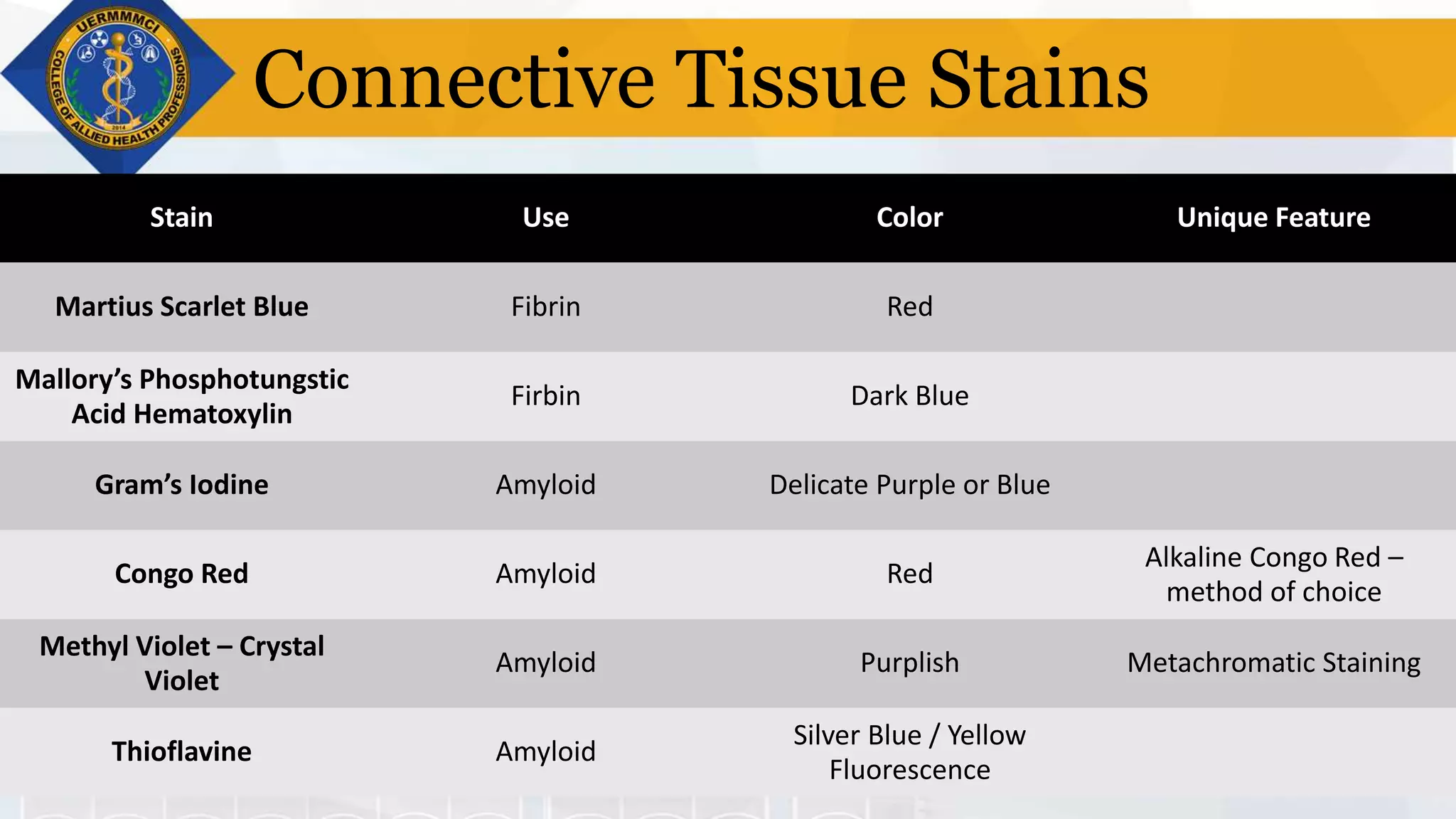
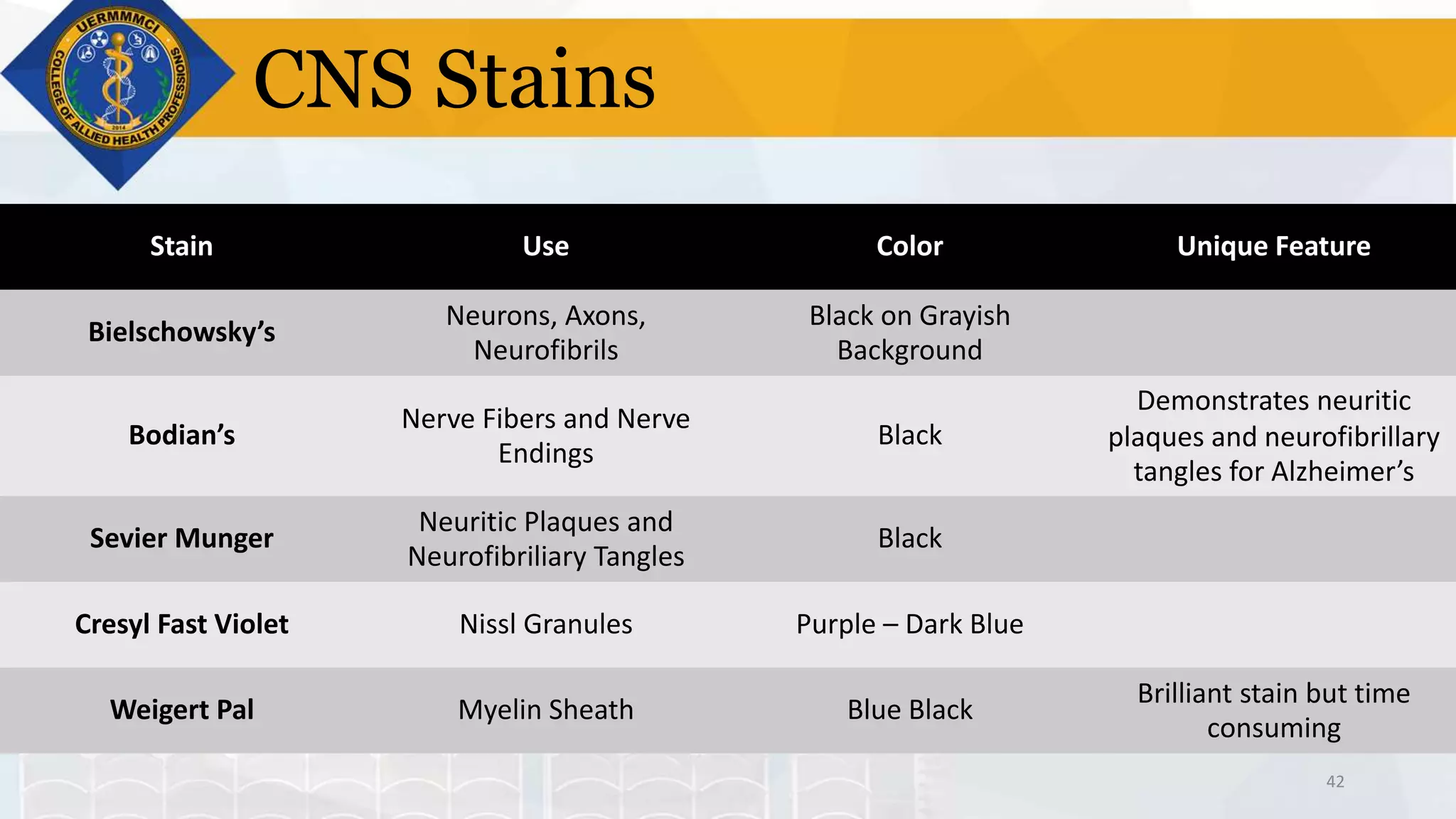
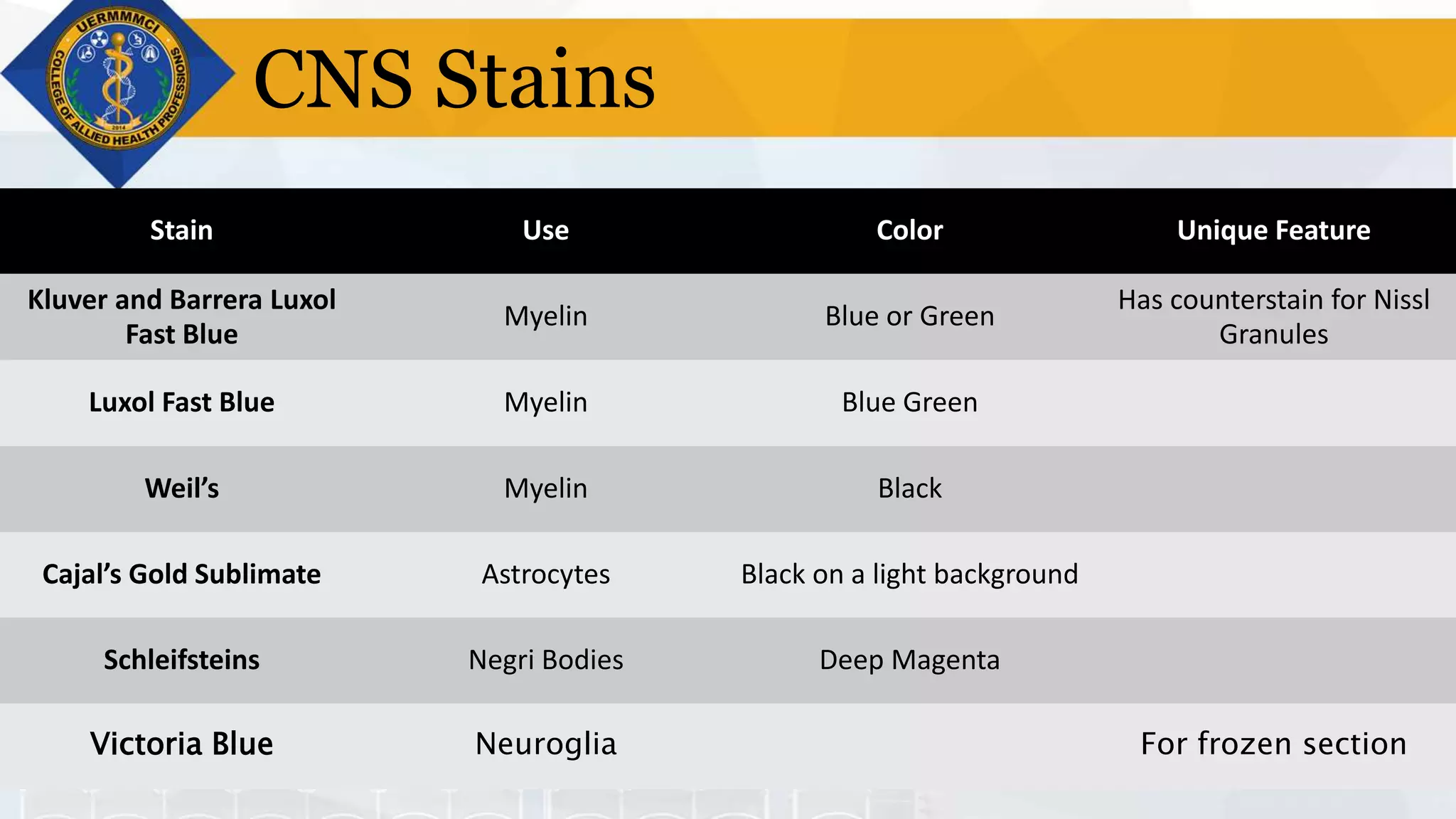
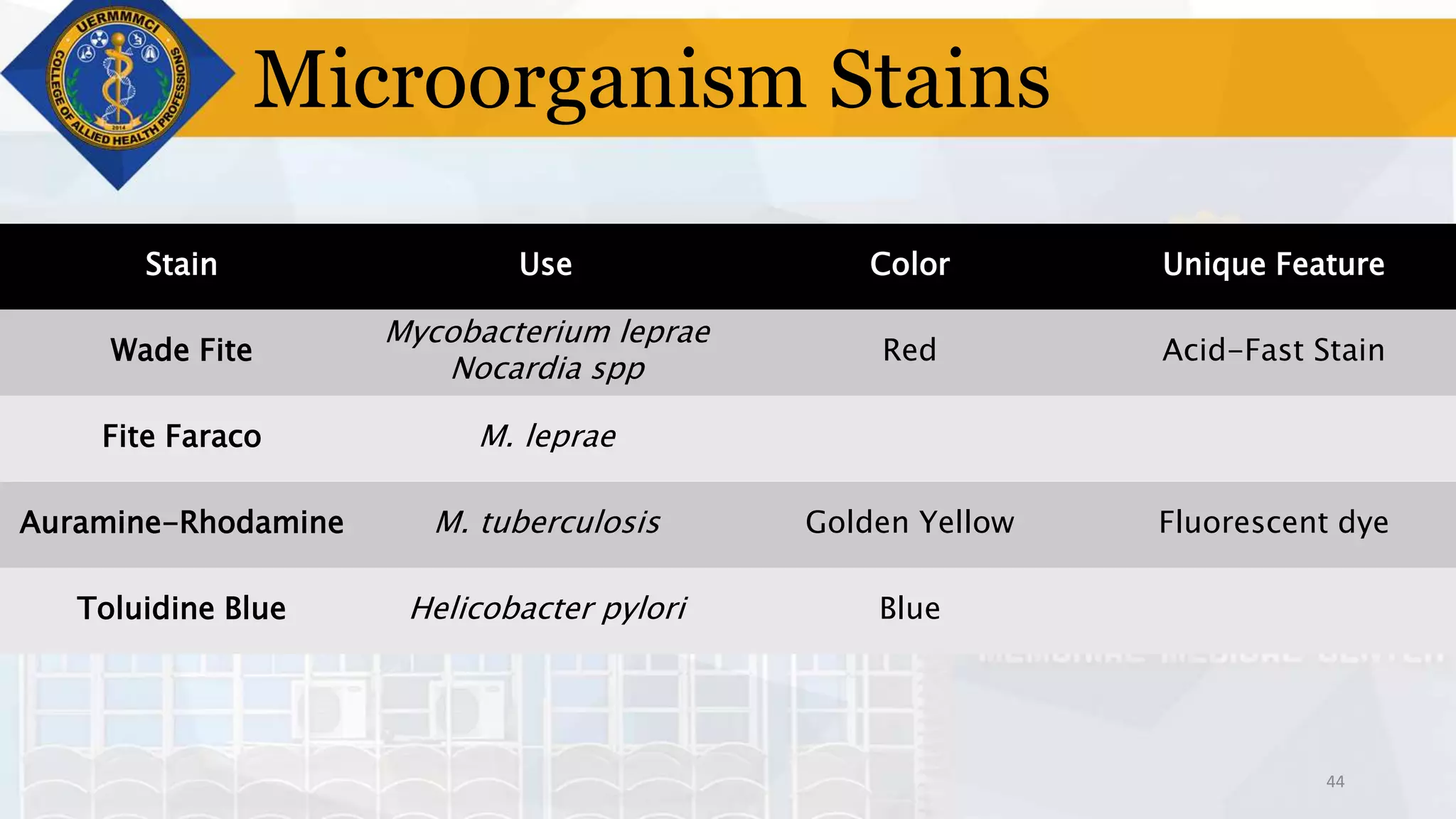
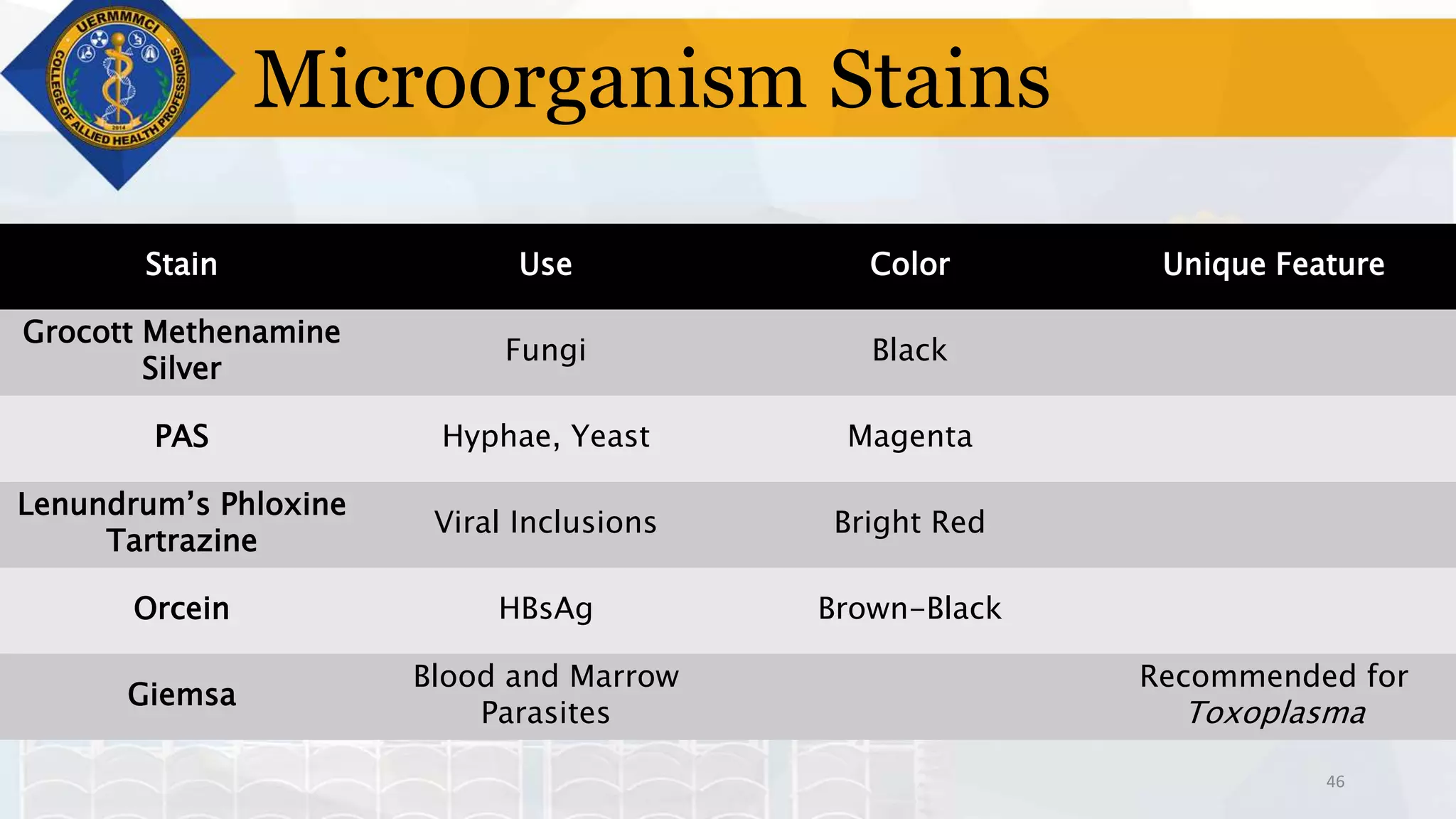
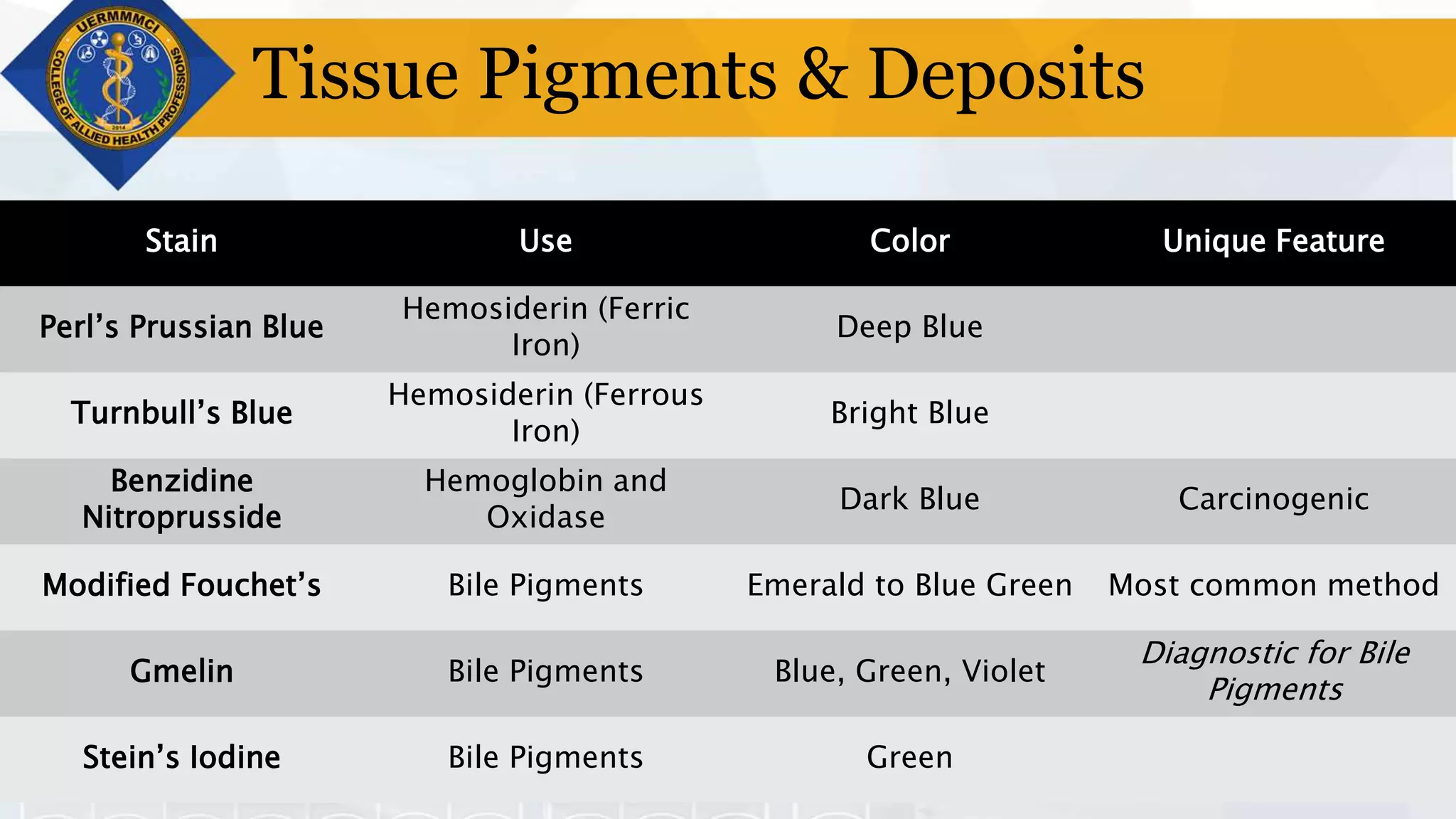
This document provides information on tissue processing and histopathologic techniques, focusing on staining. It defines staining as applying dyes to tissue sections to facilitate microscopic study. It then classifies stains based on pH, function, source, dye application technique, sequence, and color contrast. Specific staining techniques and commonly used stains are described for carbohydrates, lipids, proteins, and nucleic acids. Hematoxylin and eosin staining is explained as the most widely used staining procedure.