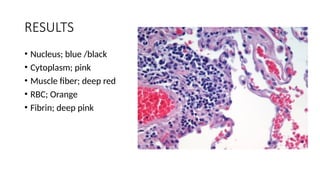
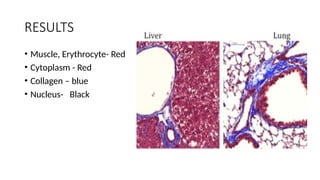
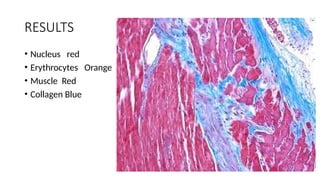
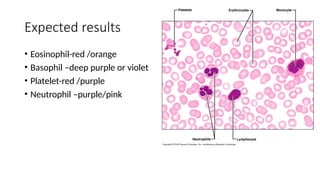
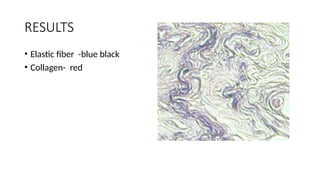
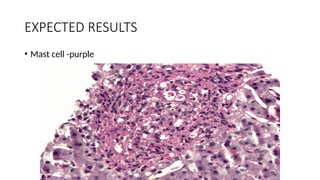
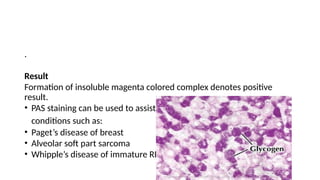
This document discusses various histological staining techniques used in tissue examination, detailing key stains such as hematoxylin and eosin, Masson's trichrome, and silver stains. It outlines their principles, procedures, and expected results, emphasizing the differentiation of cell structures and types. The document serves as a comprehensive guide for performing histological stains and interpreting the results for diagnostic purposes.