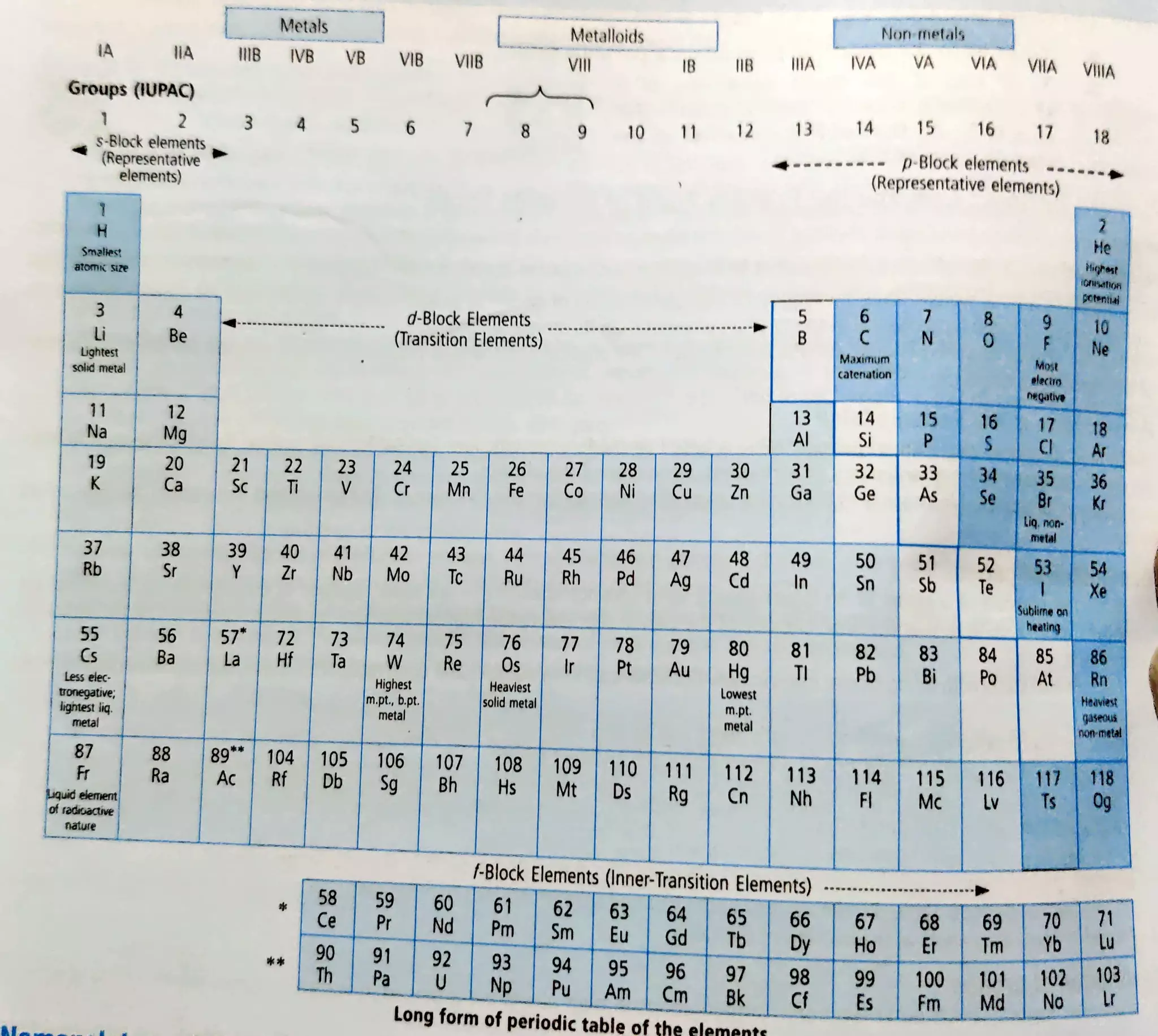
This document provides information about the periodic table, including its organization and block structure. It describes the electronic configuration and properties of elements in the s-block, p-block, d-block, and f-block. The s-block contains alkali and alkaline earth metals. The p-block contains nonmetals and metalloids. The d-block contains transition metals. The f-block contains lanthanides and actinides. Each block has characteristic trends in properties corresponding to the orbital being filled.