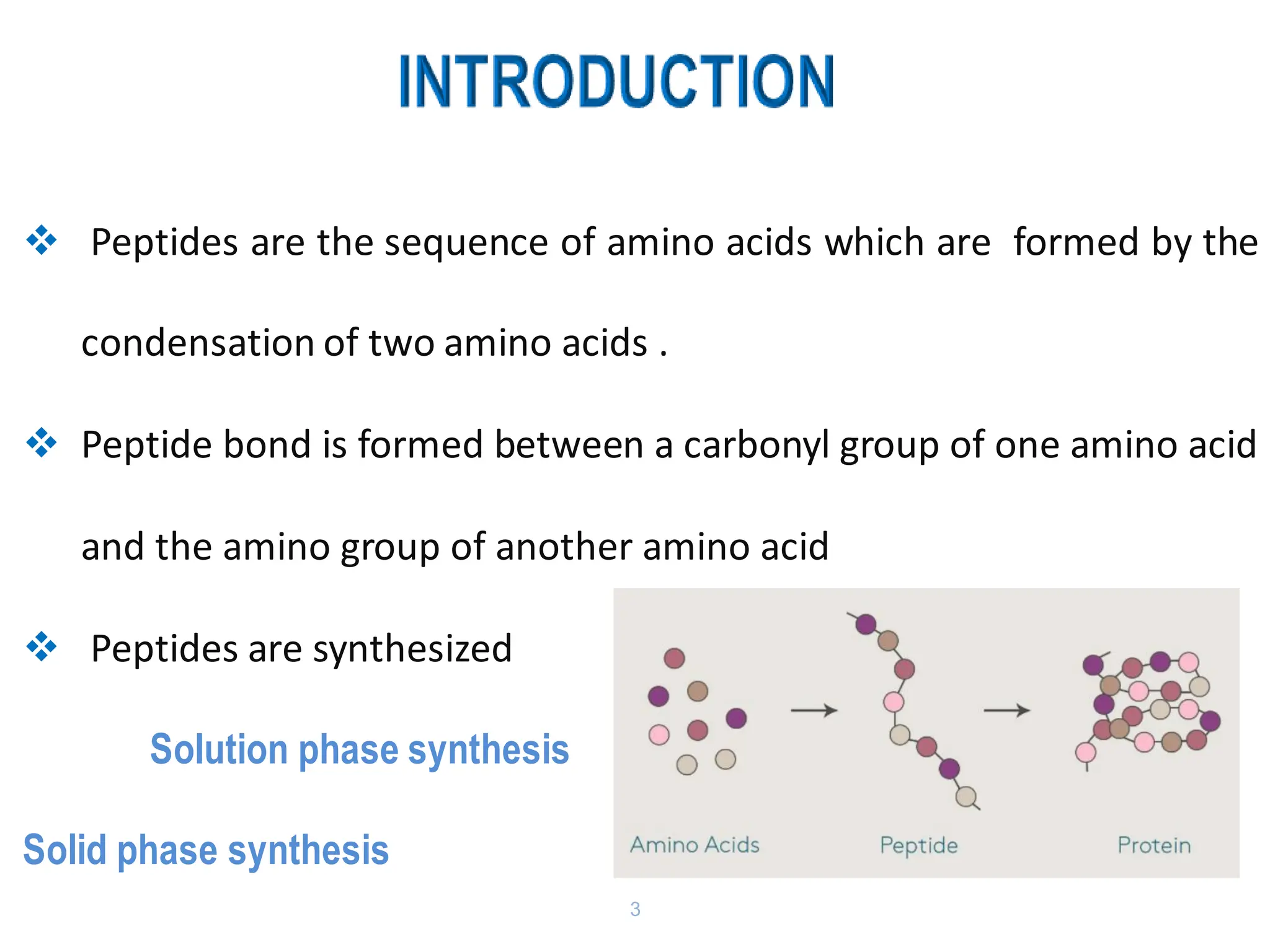
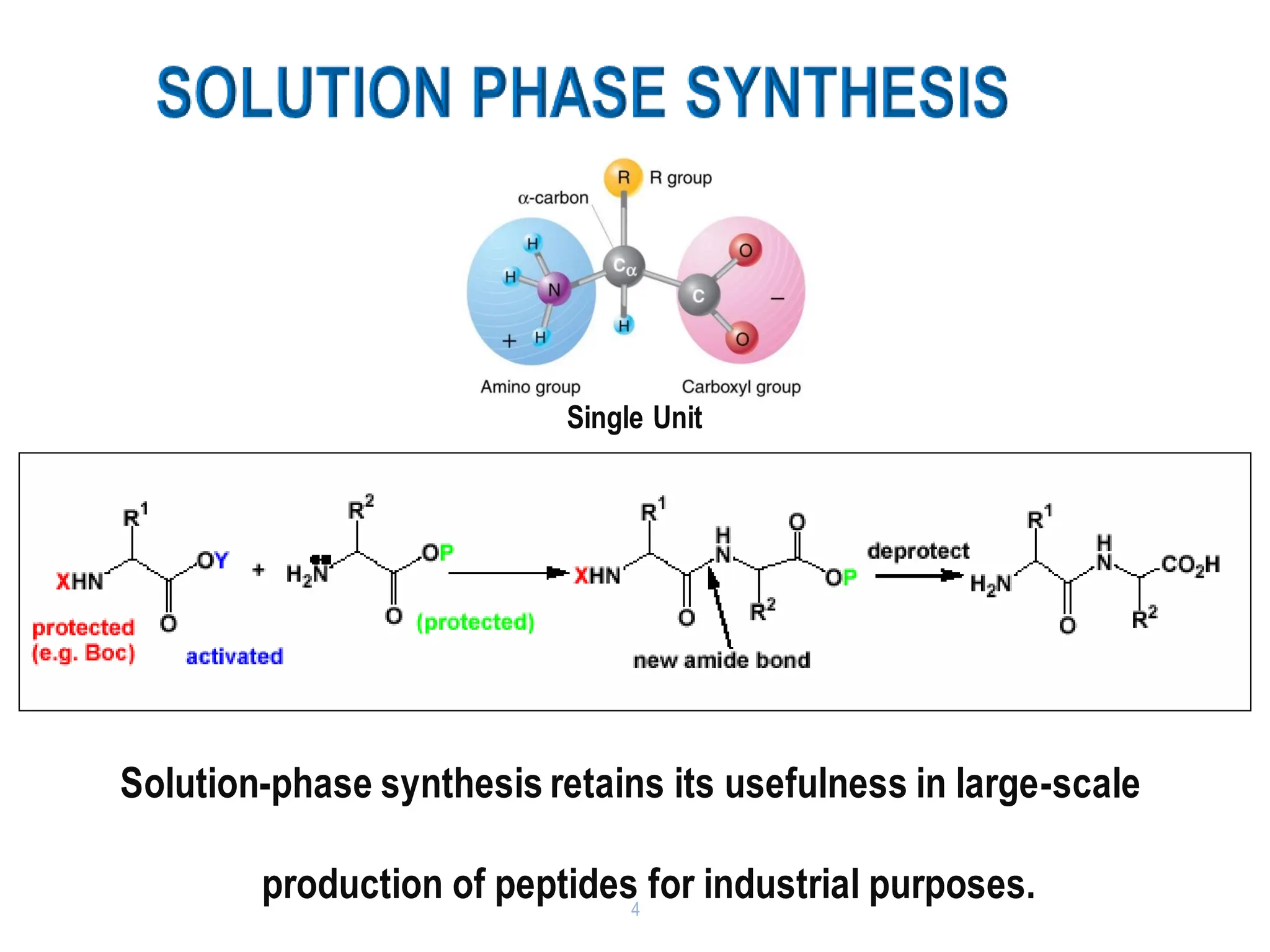
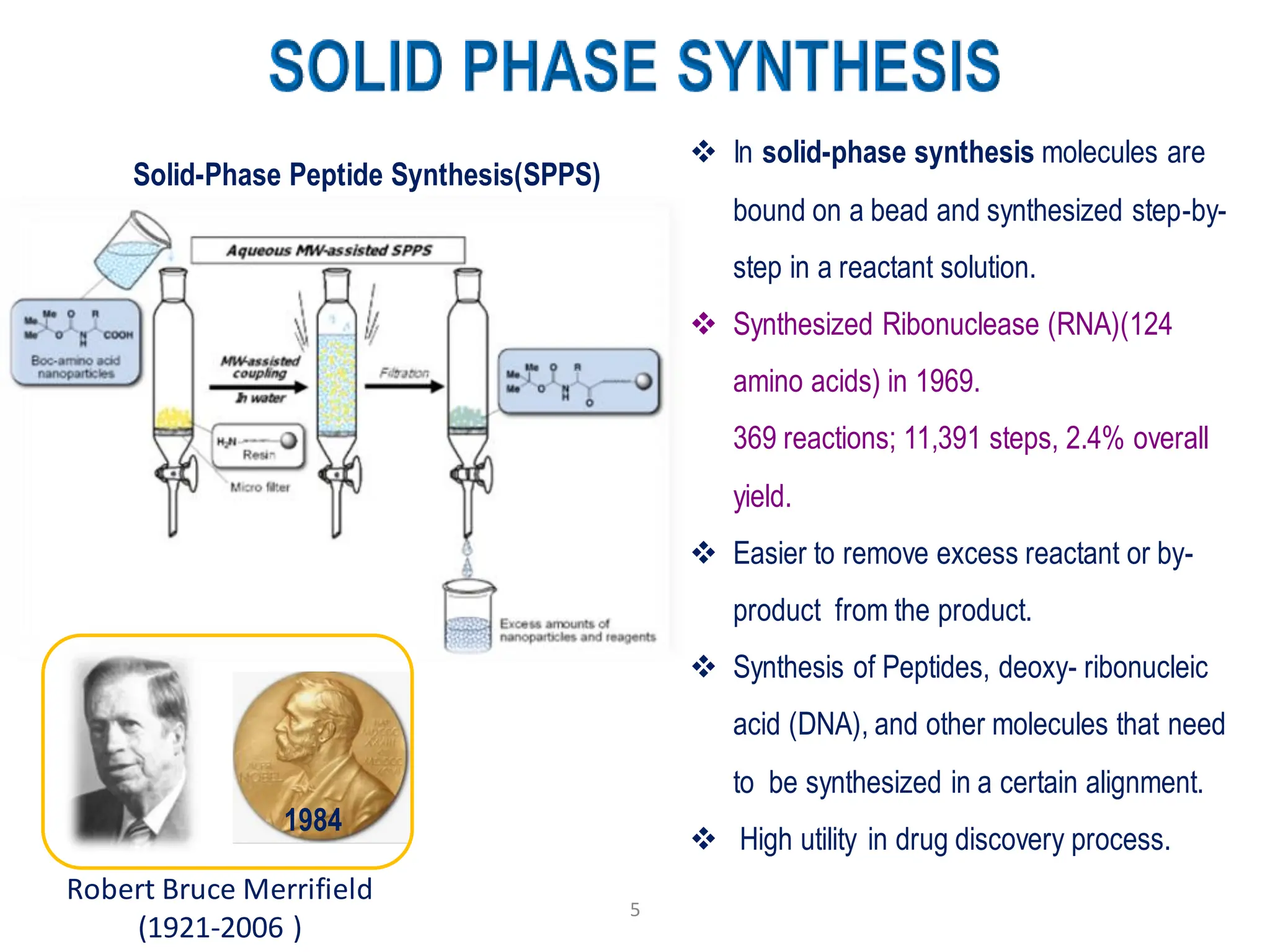
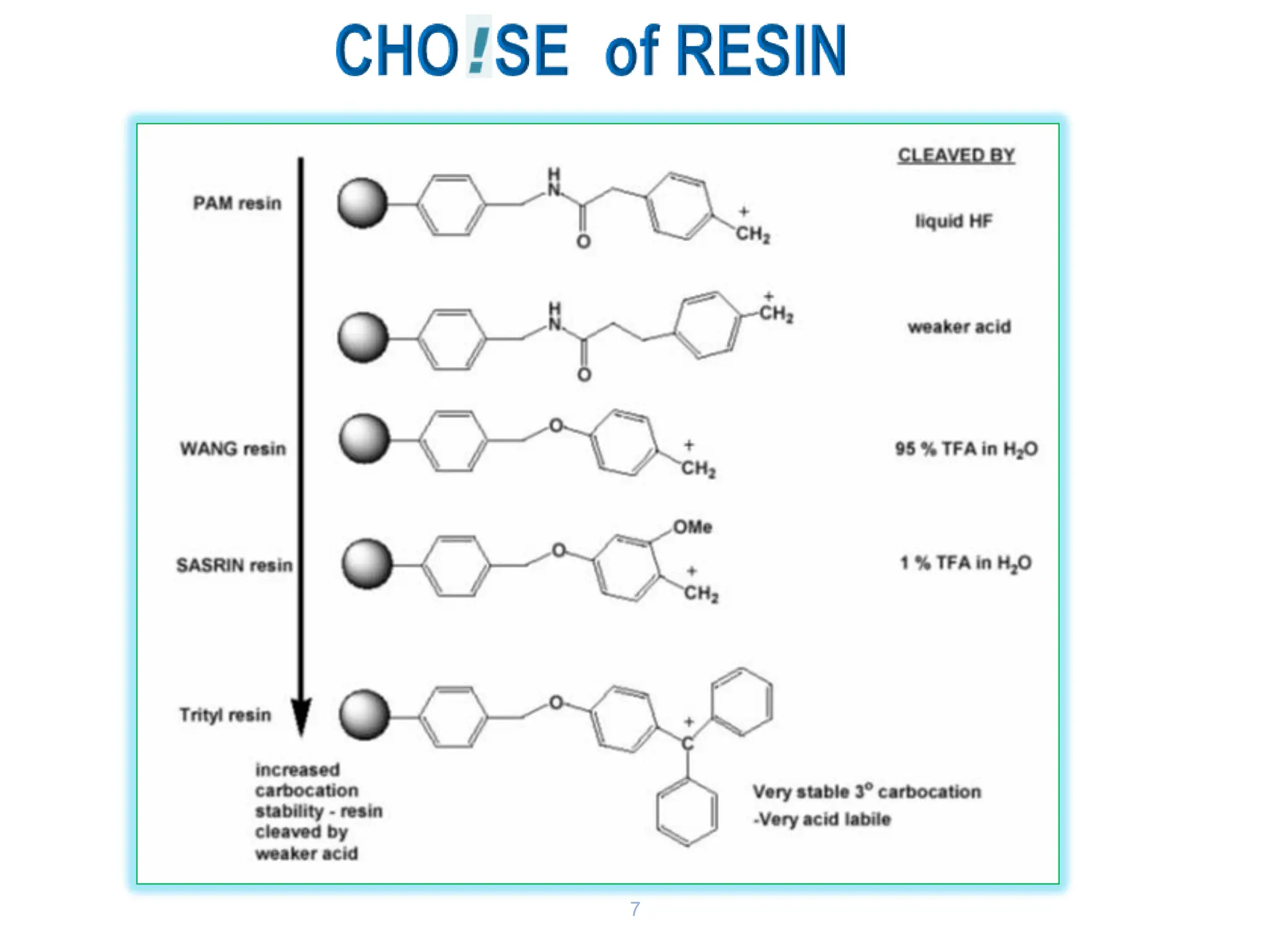
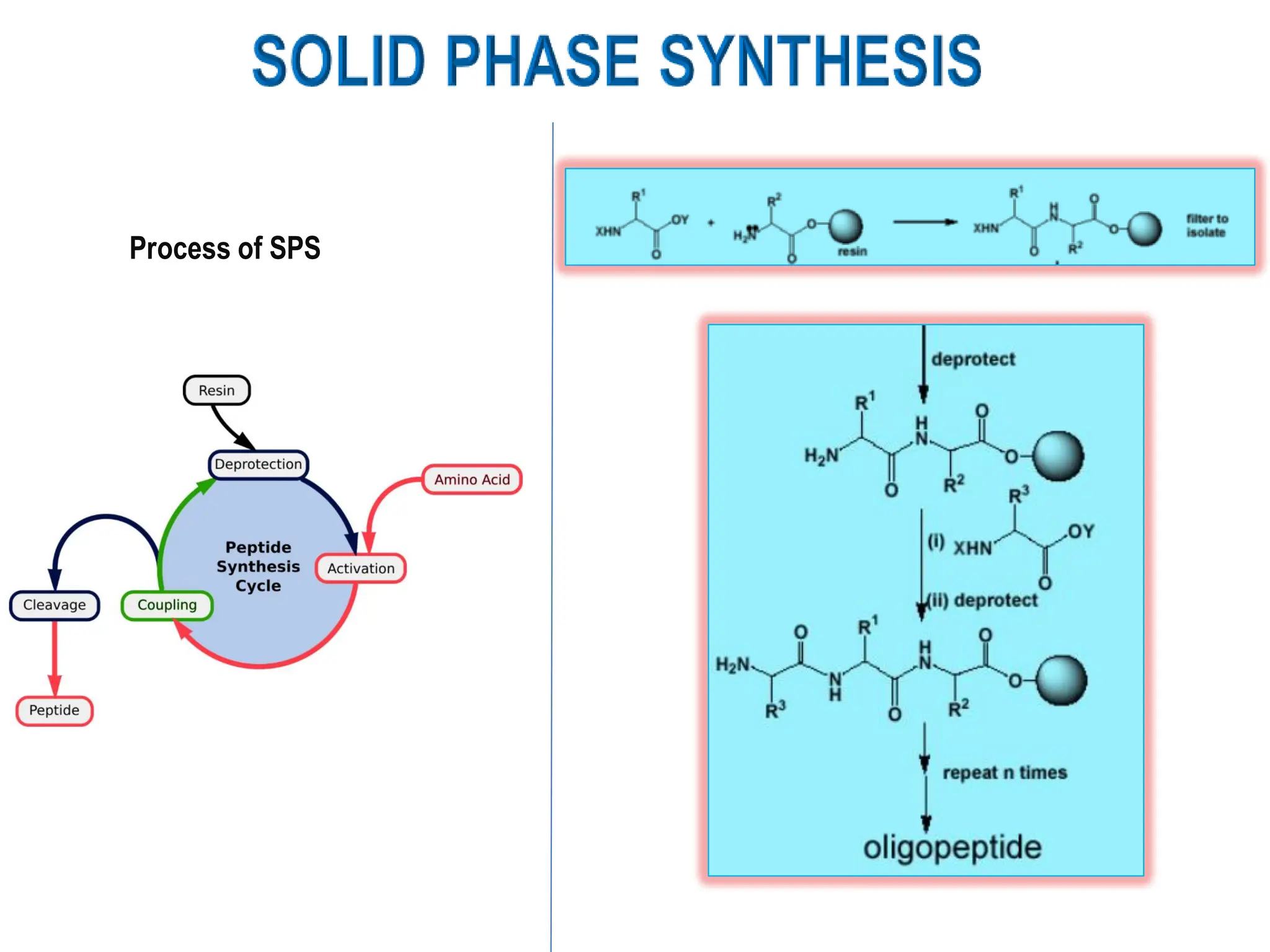
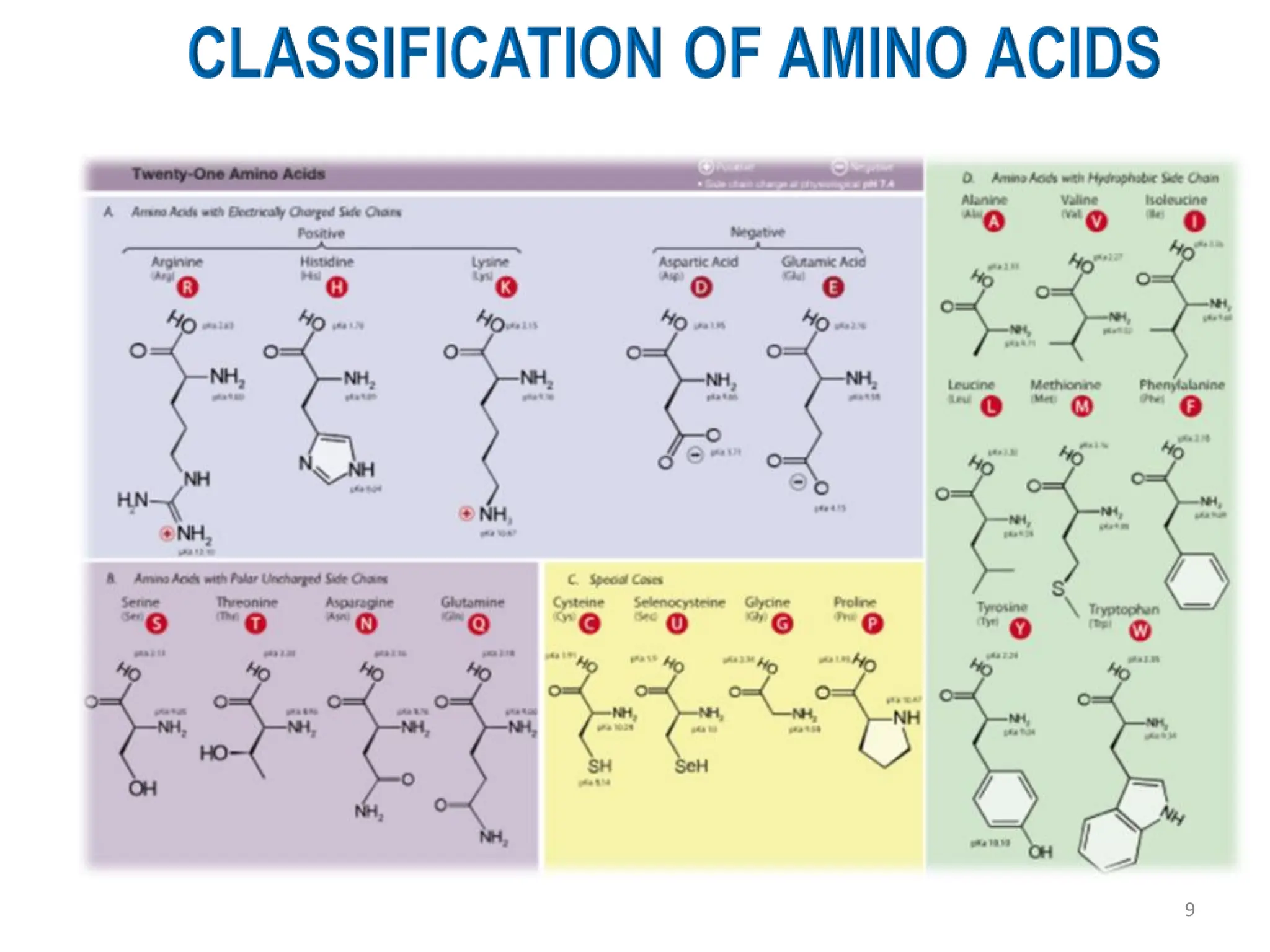
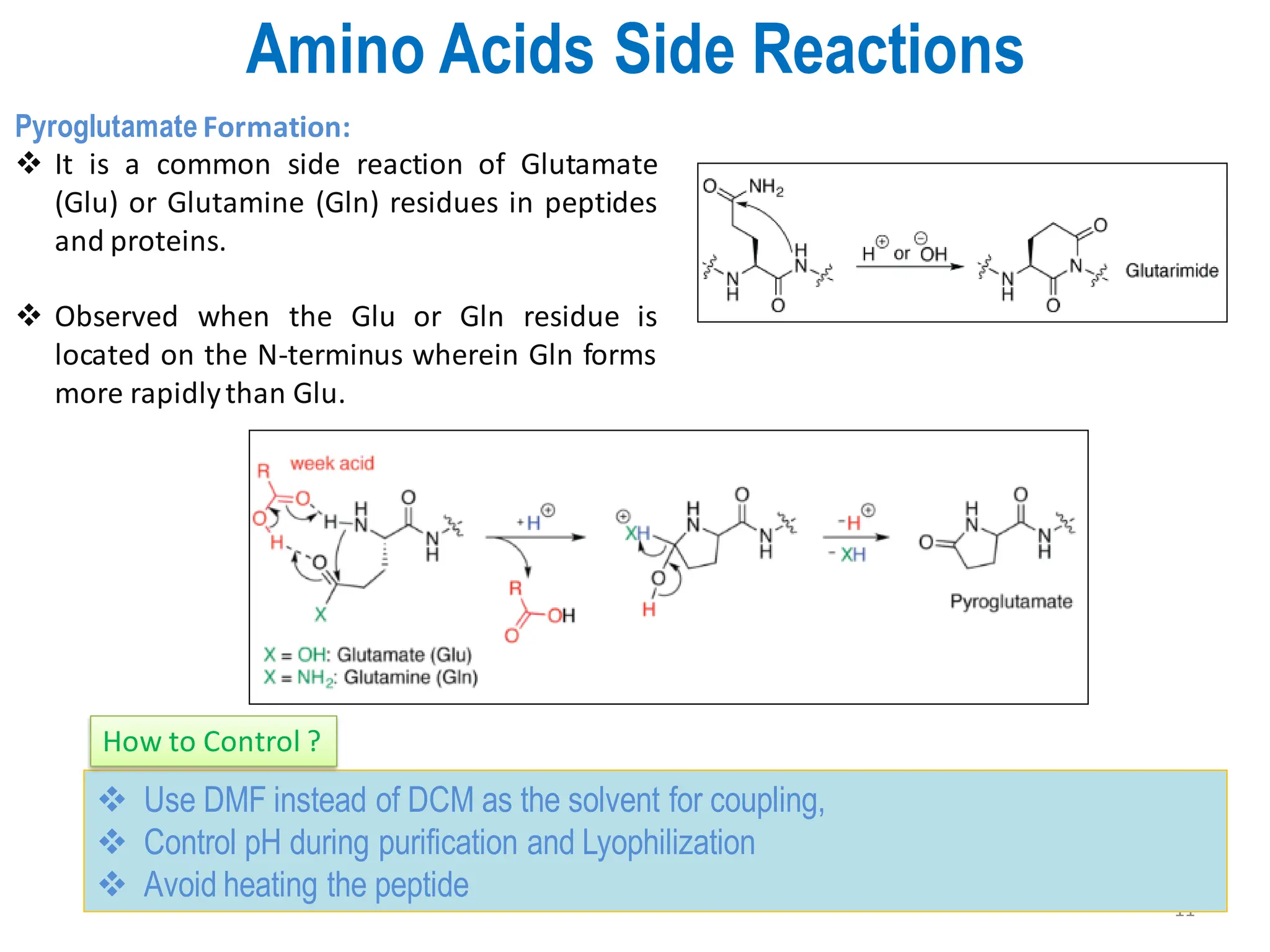
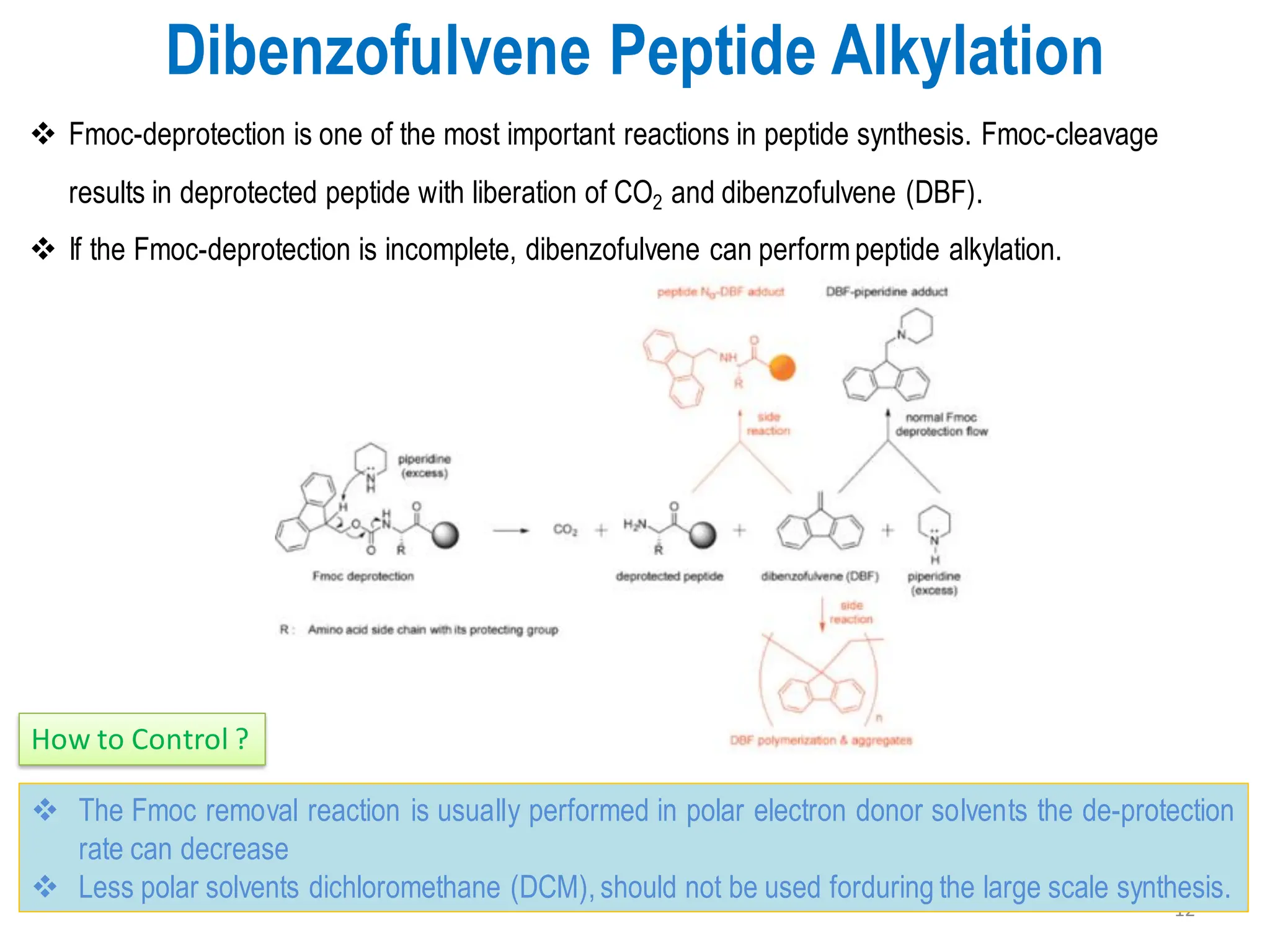
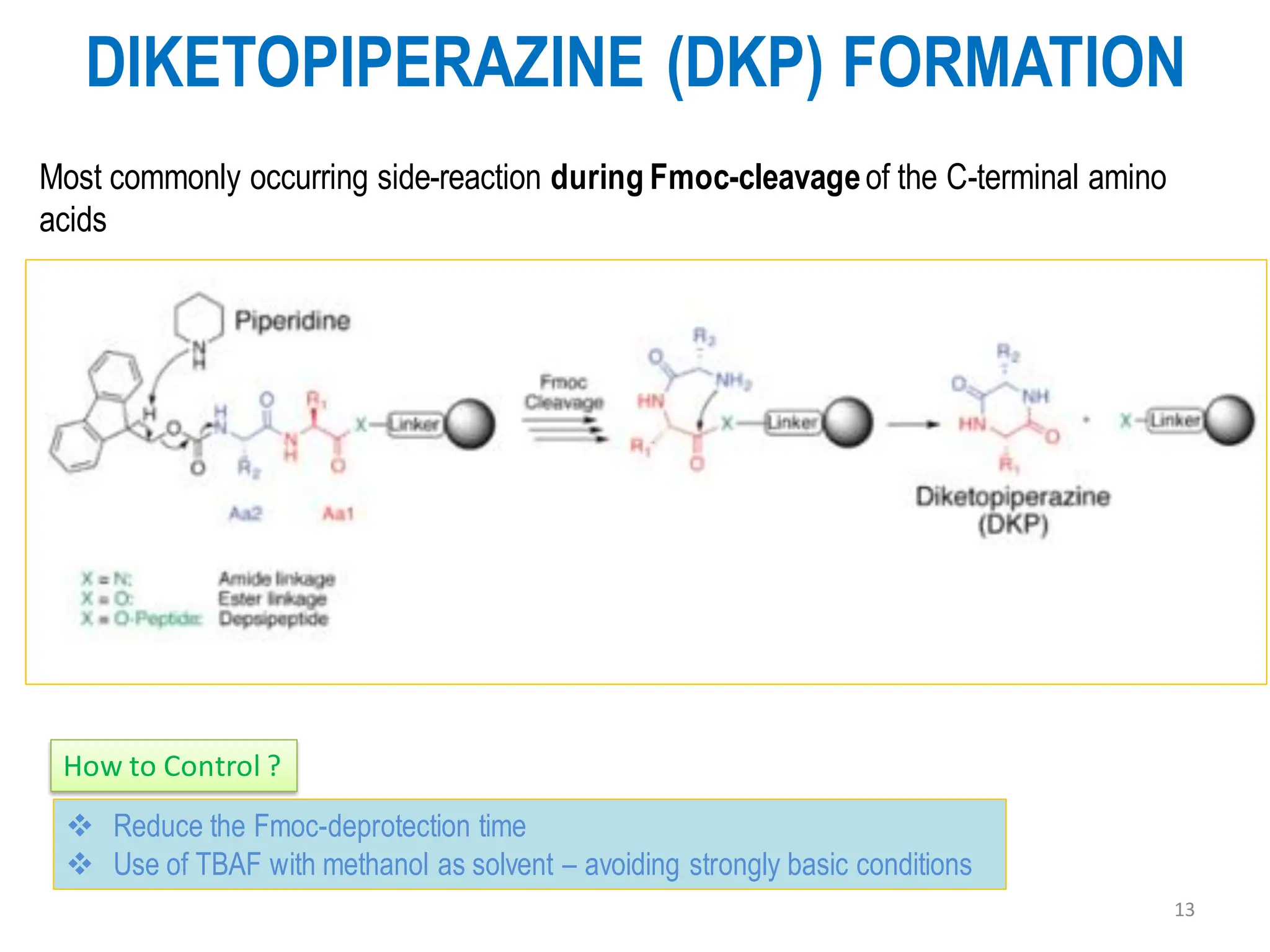
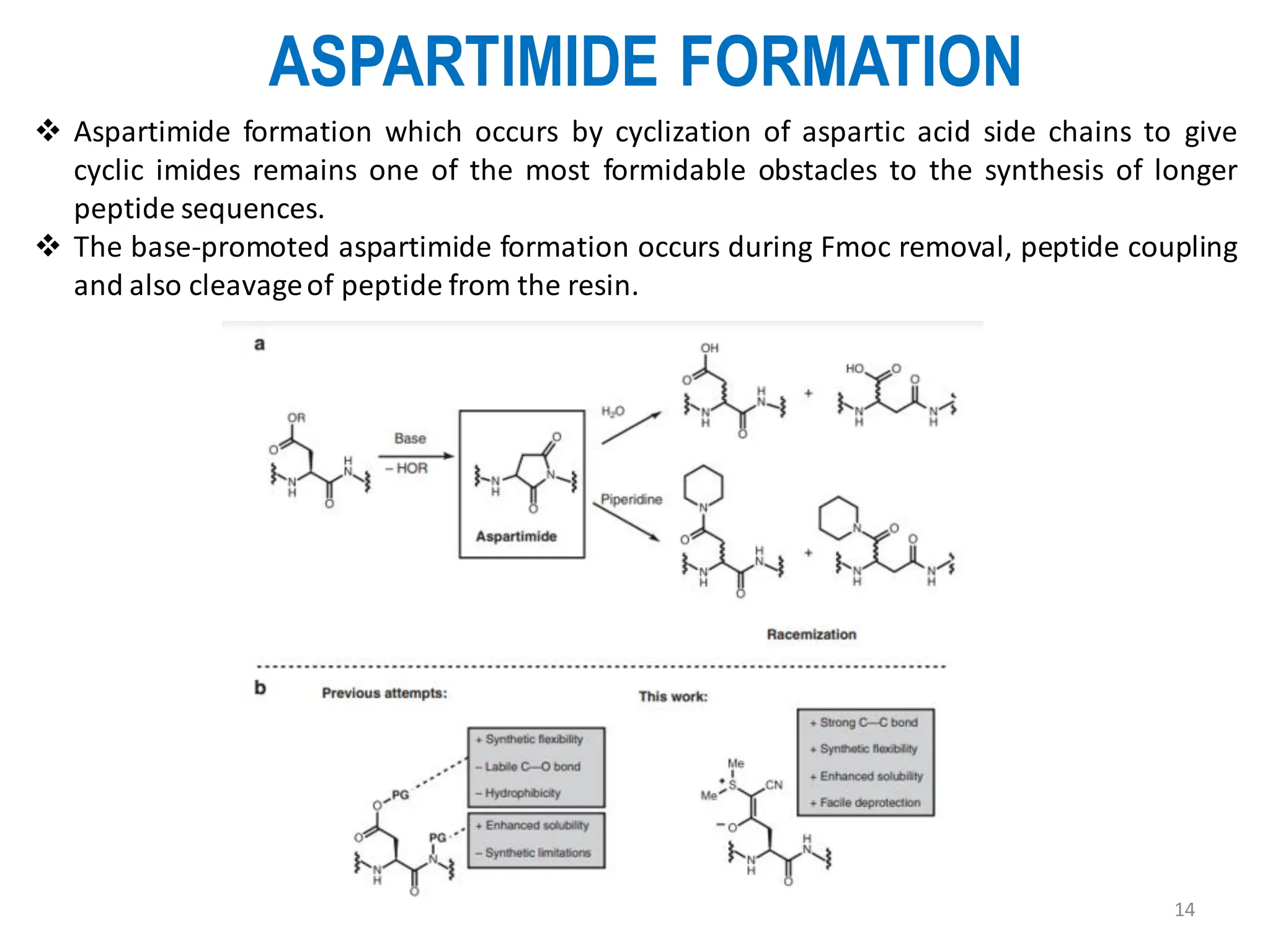
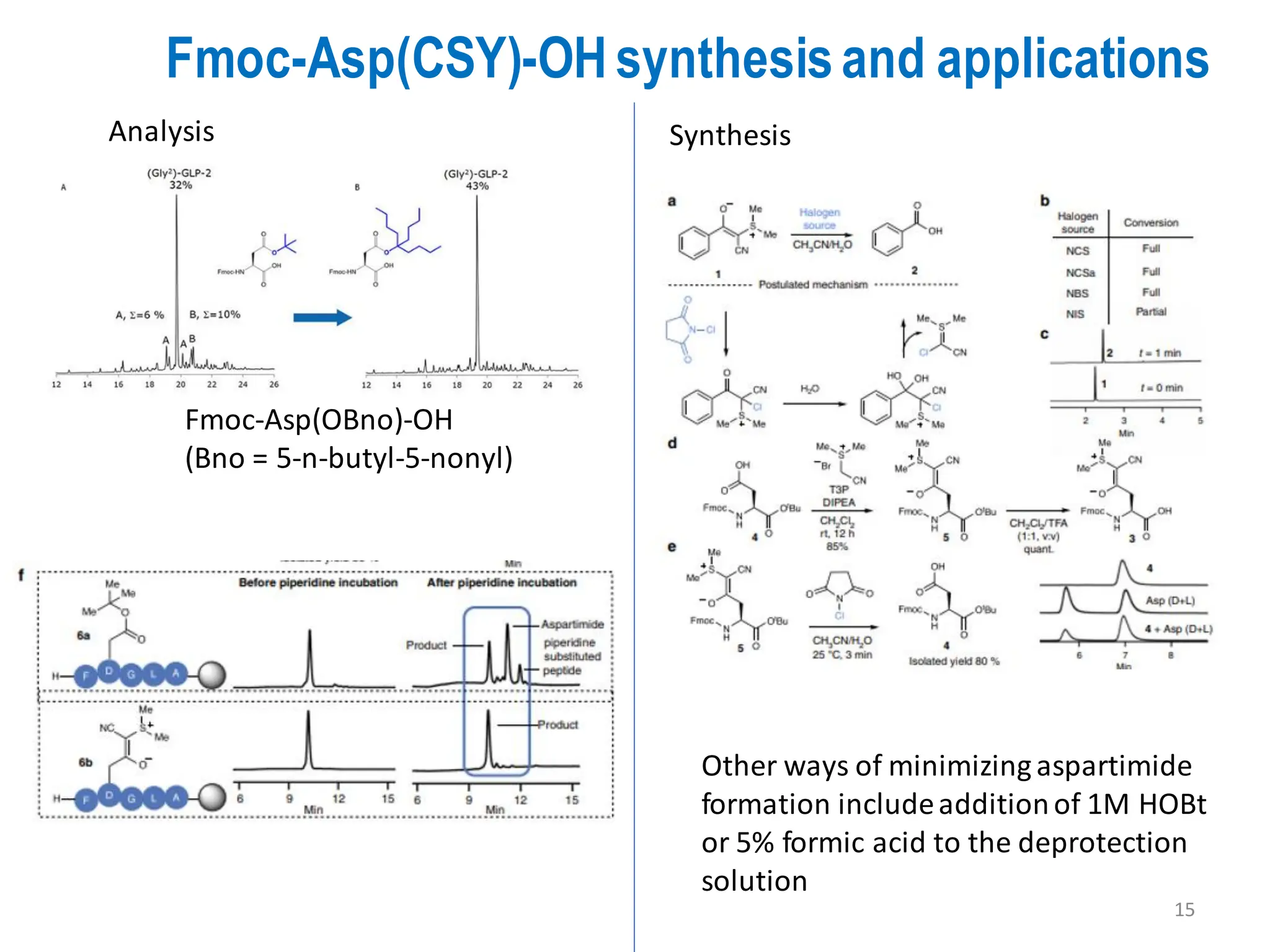
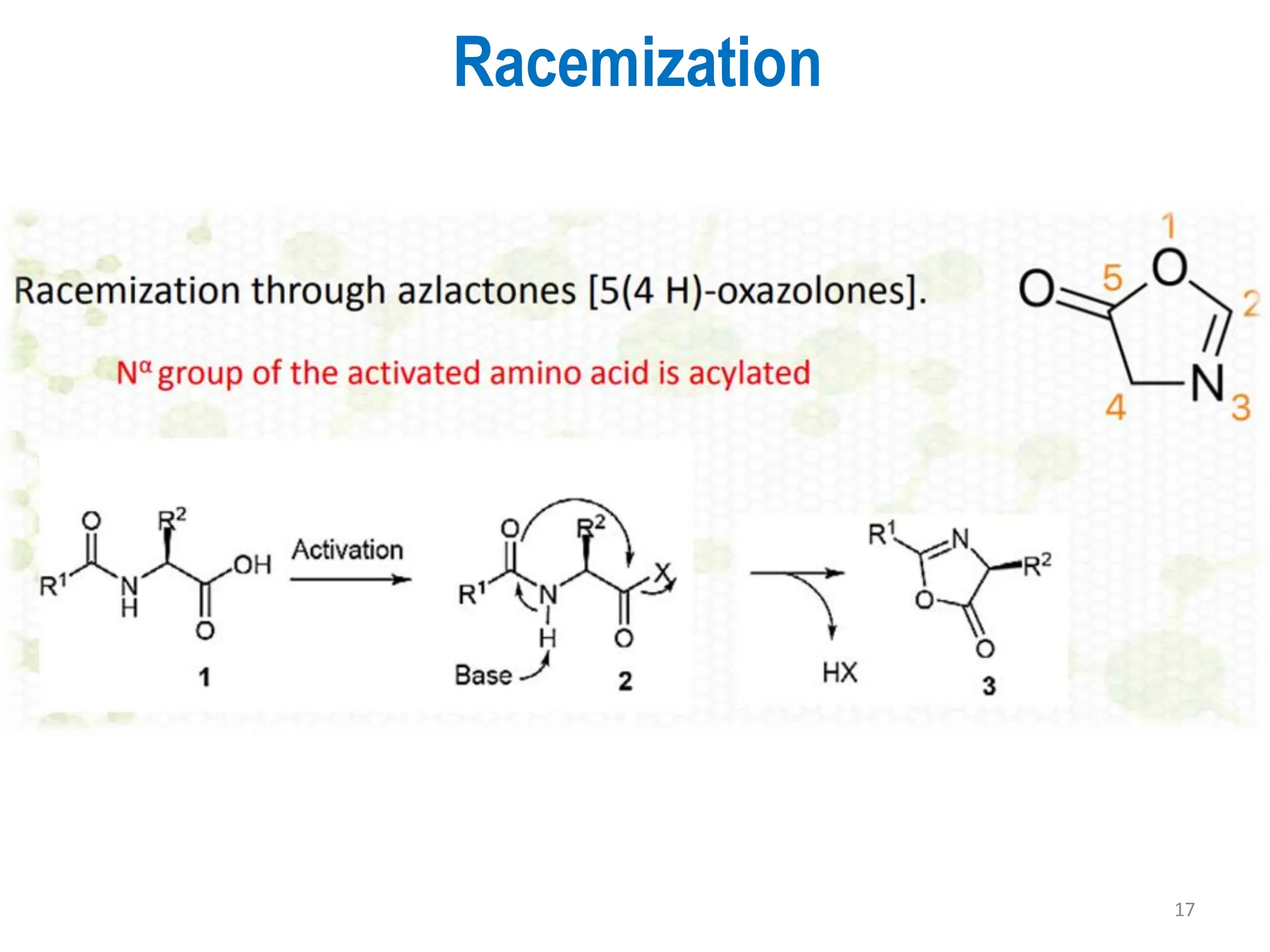
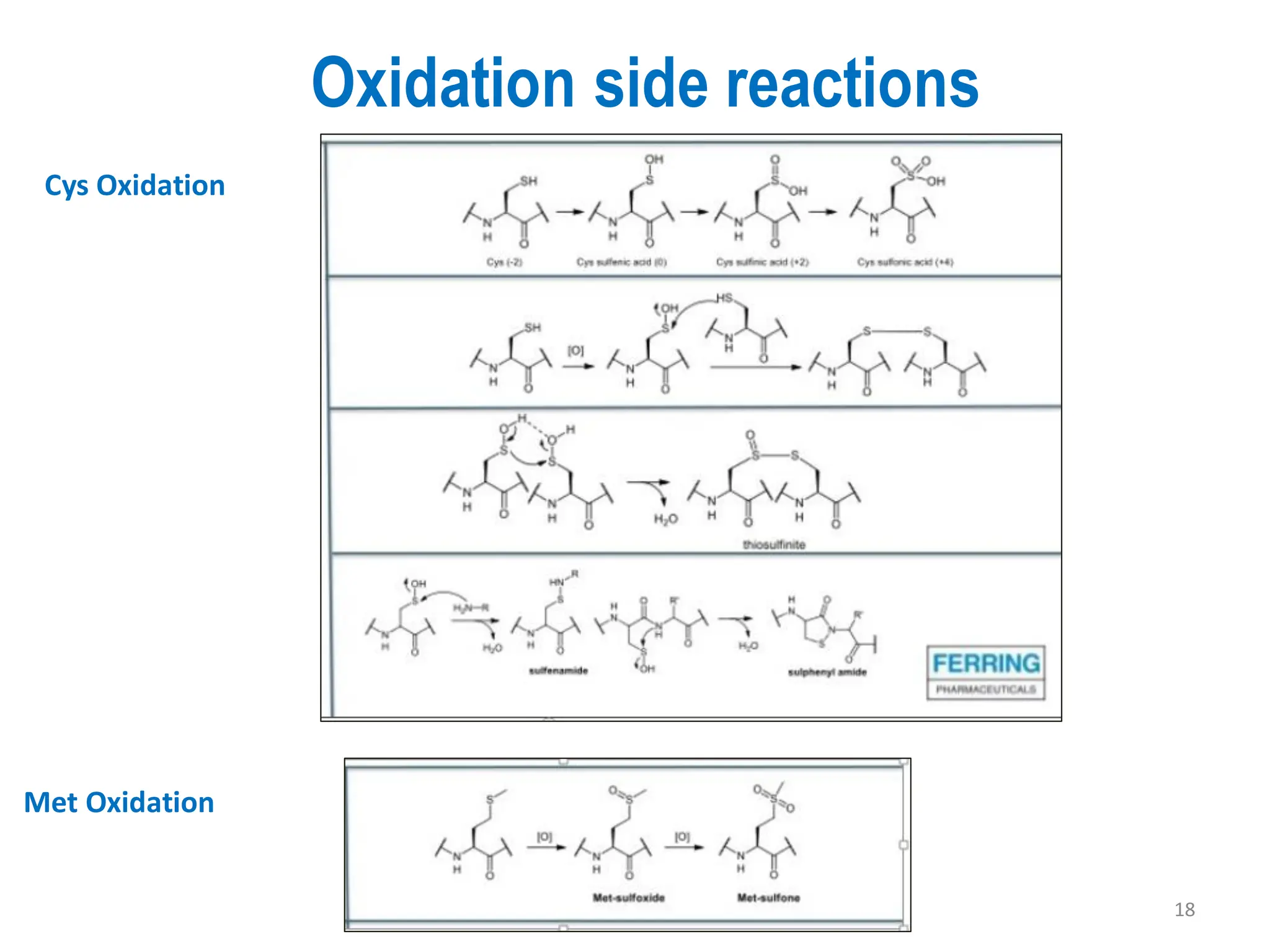
The presentation on "Side Reactions in Peptide Synthesis" explores peptide synthesis through solution-phase and solid-phase methods, focusing on solid-phase peptide synthesis (SPPS) and its use of polystyrene resins. It highlights common side reactions, such as pyroglutamate formation, aspartimide formation, and racemization, and discusses control strategies like solvent selection. SPPS is presented as a robust, green chemistry technique for synthesizing peptides, DNA, and combinatorial molecules, despite challenges from side chain degradation.