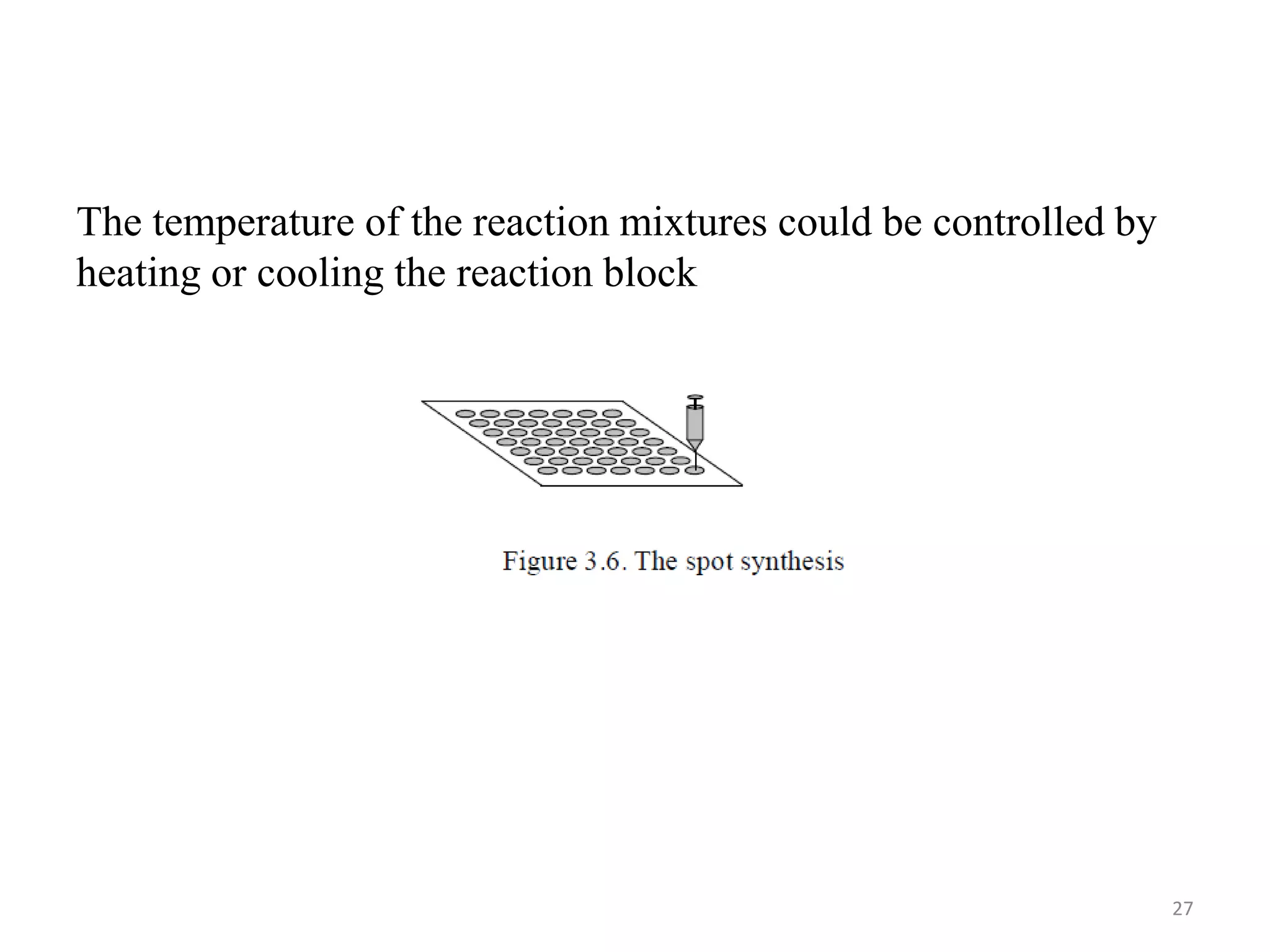
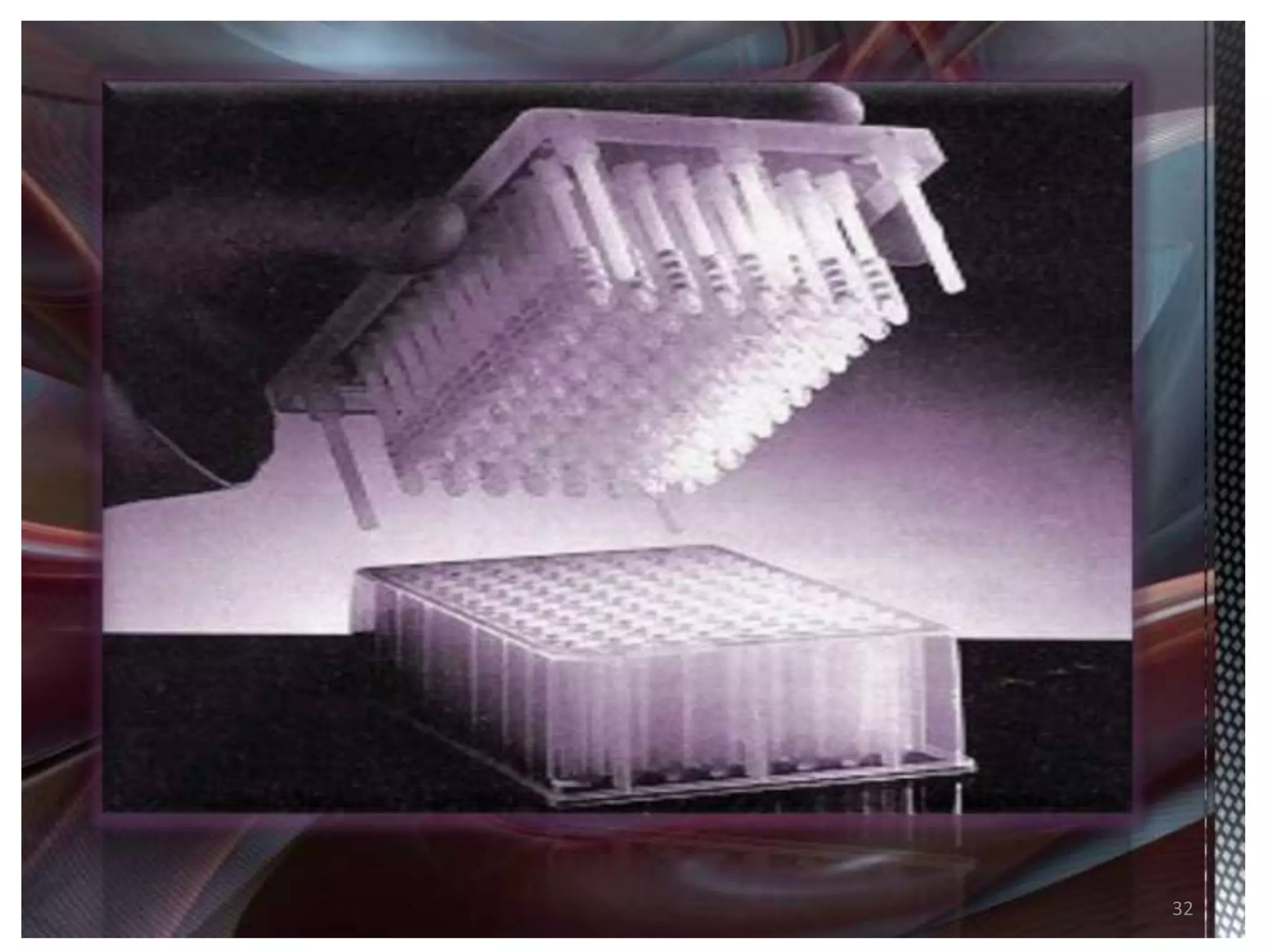
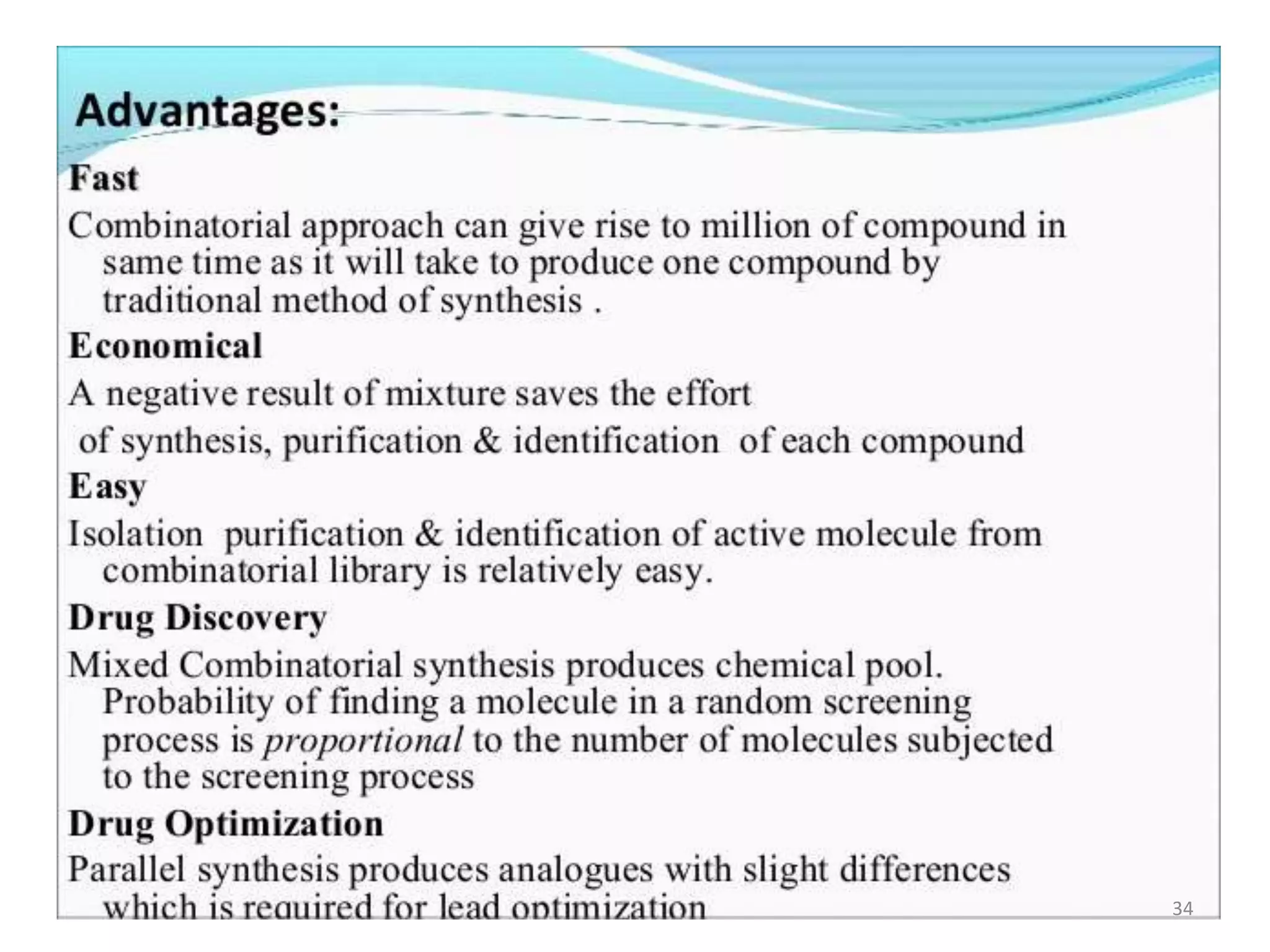
1) Combinatorial chemistry techniques allow for the parallel synthesis of multiple chemical compounds. This involves dividing chemical reactions into separate vessels to allow many reactions to occur simultaneously, saving time over sequential synthesis.
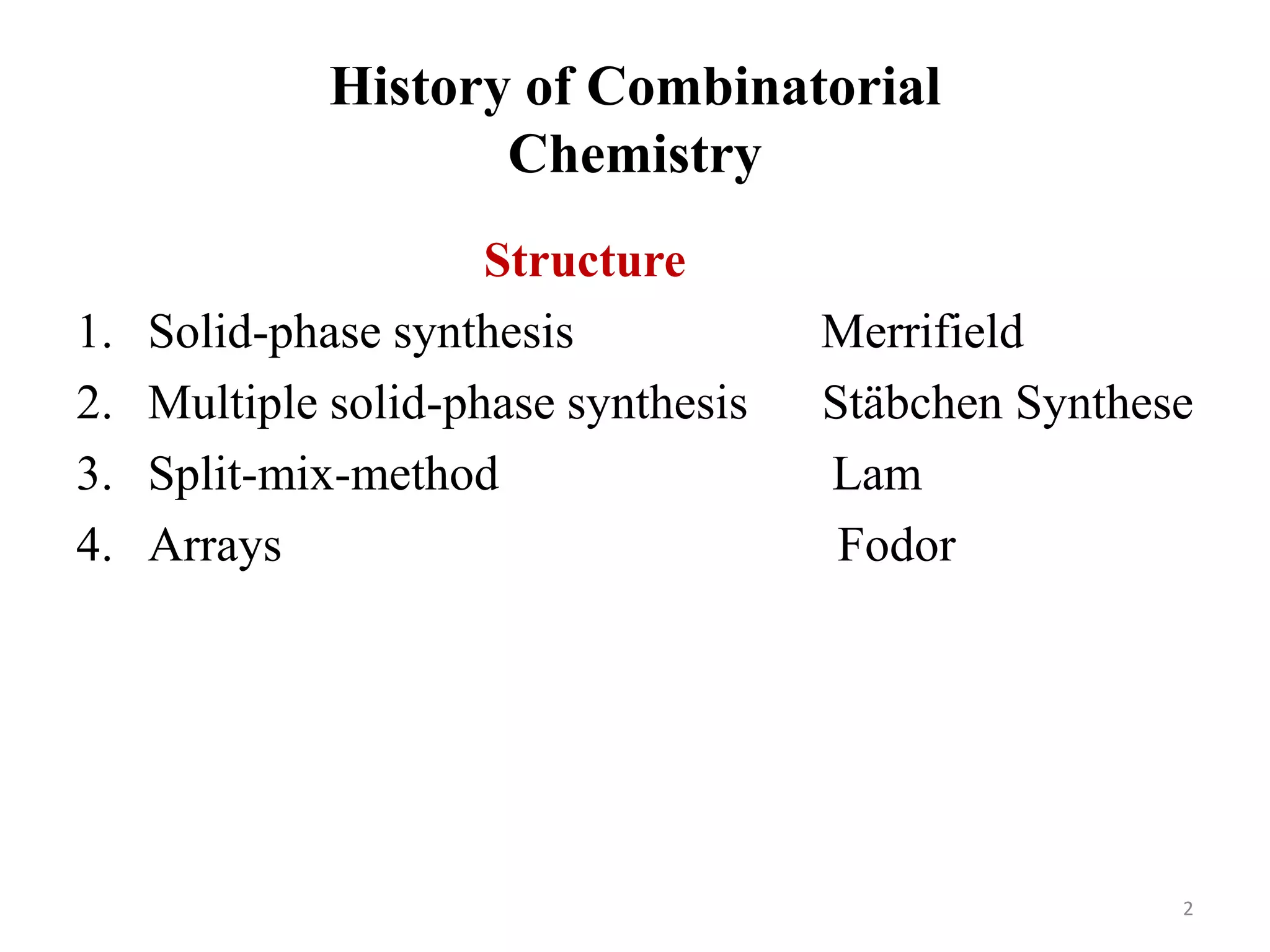
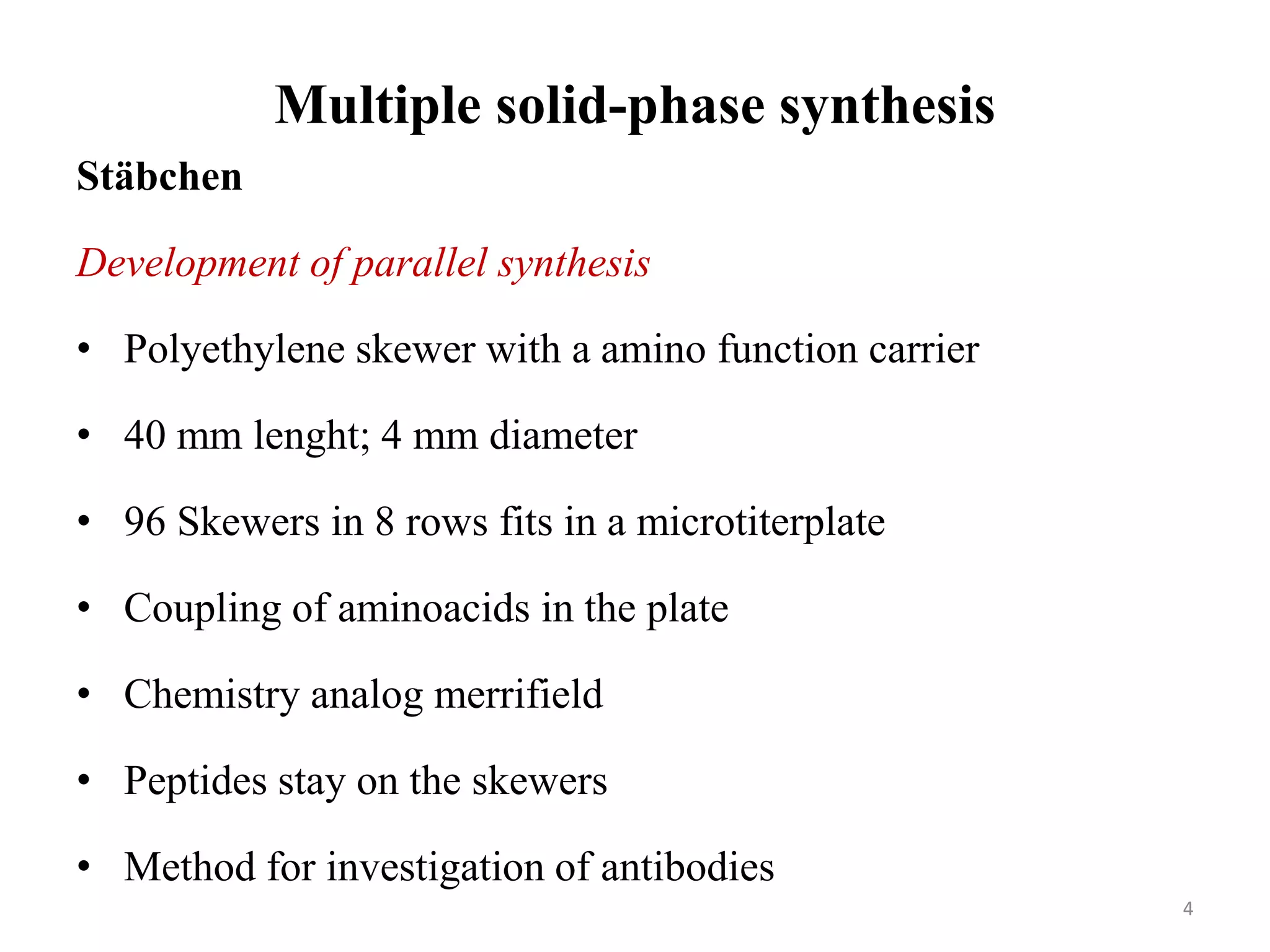
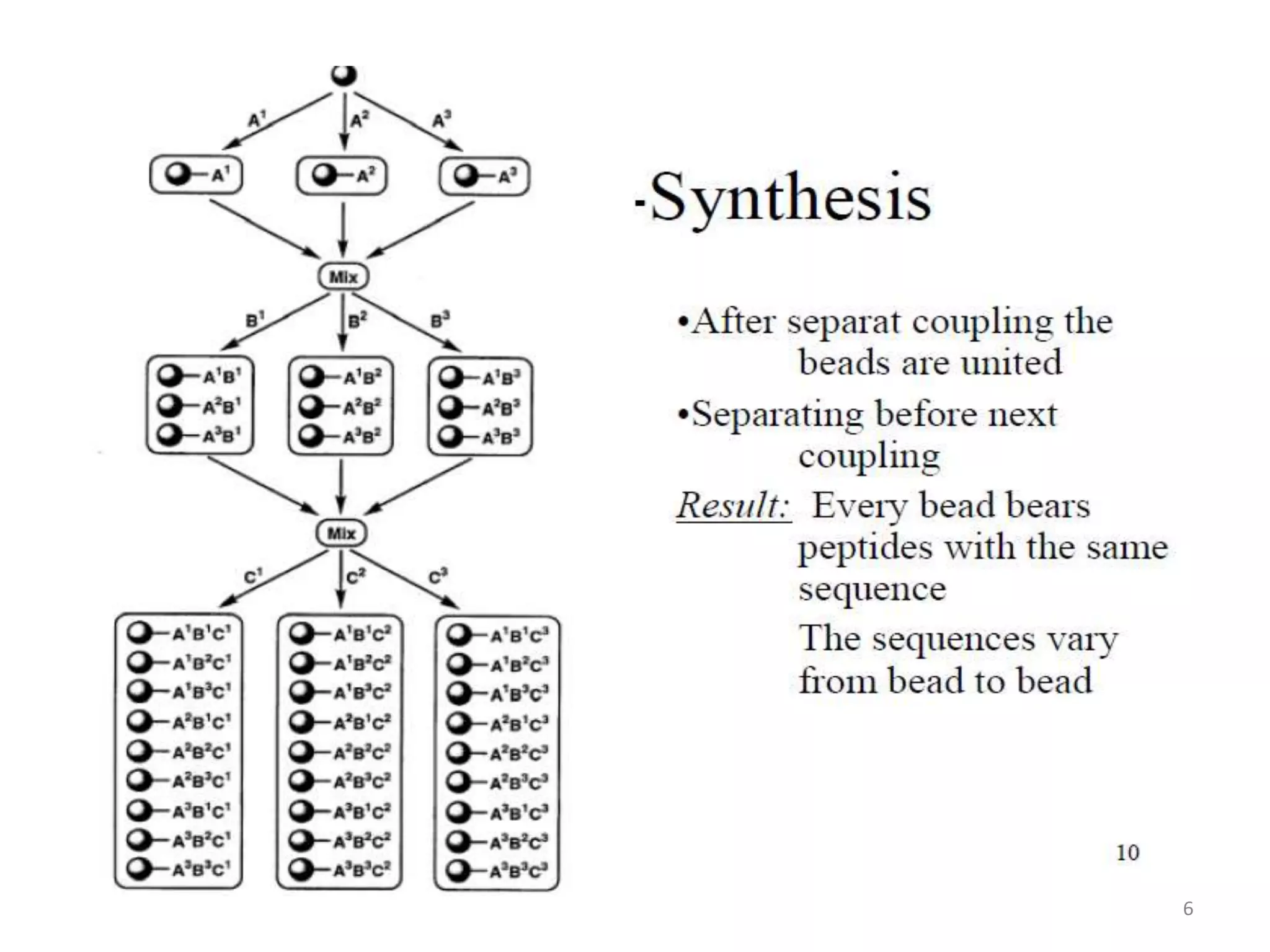
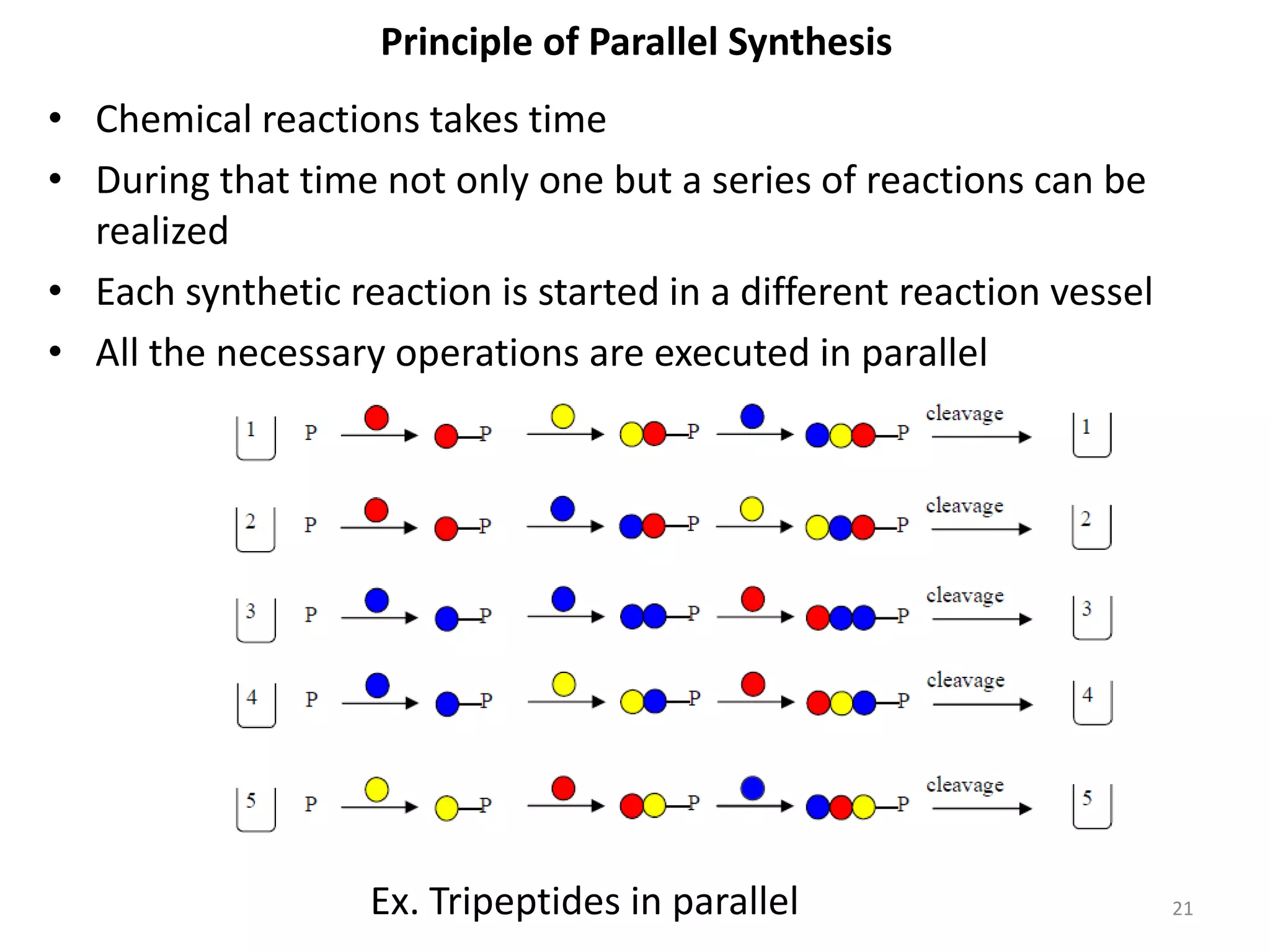
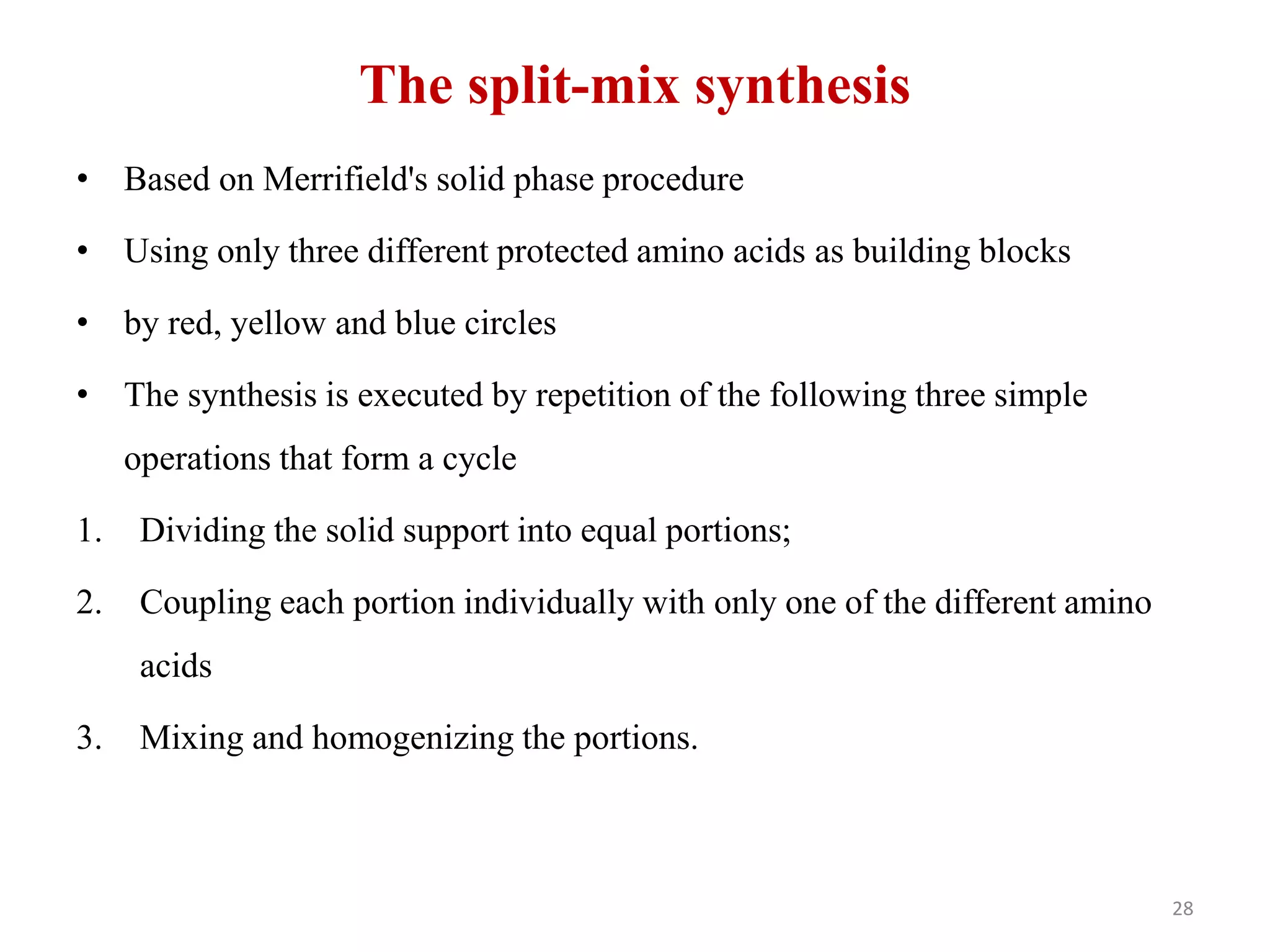
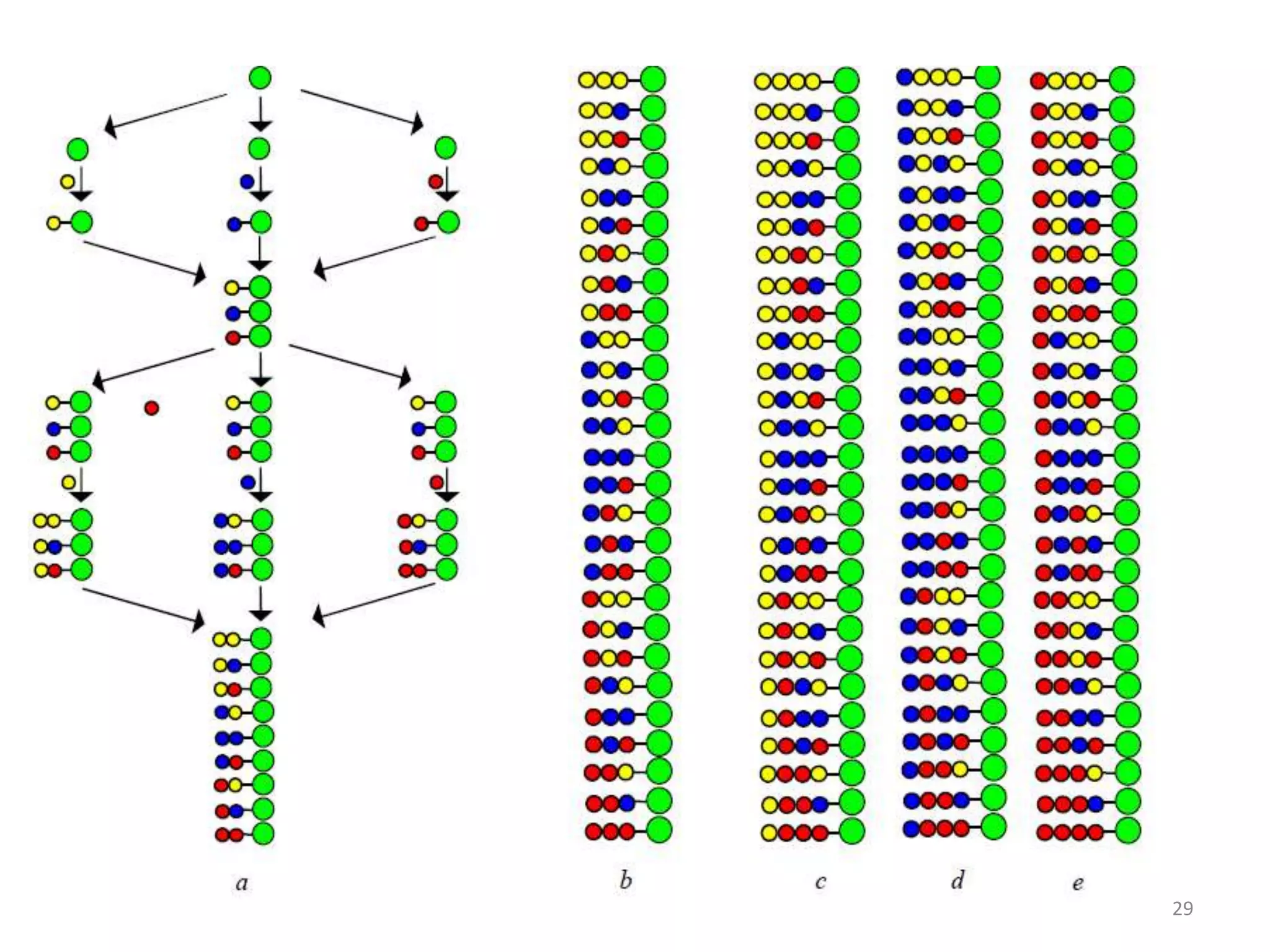
2) Some key developments in parallel synthesis techniques include Merrifield's solid phase peptide synthesis using resin beads, Stabchen's use of polyethylene rods for parallel peptide synthesis, and Lam's split-mix method which uses repetition of dividing, reacting, and recombining steps to efficiently generate compound libraries.
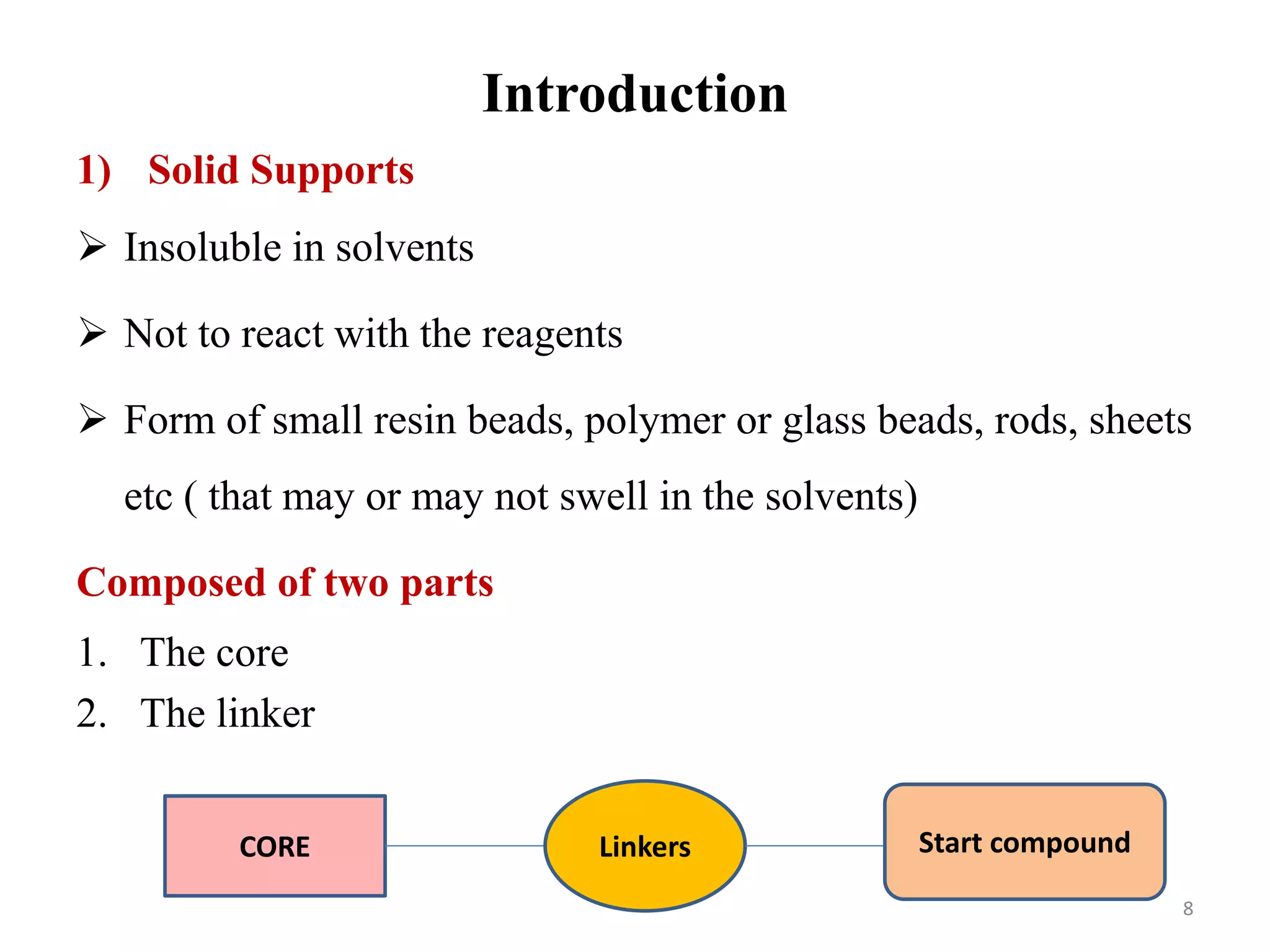
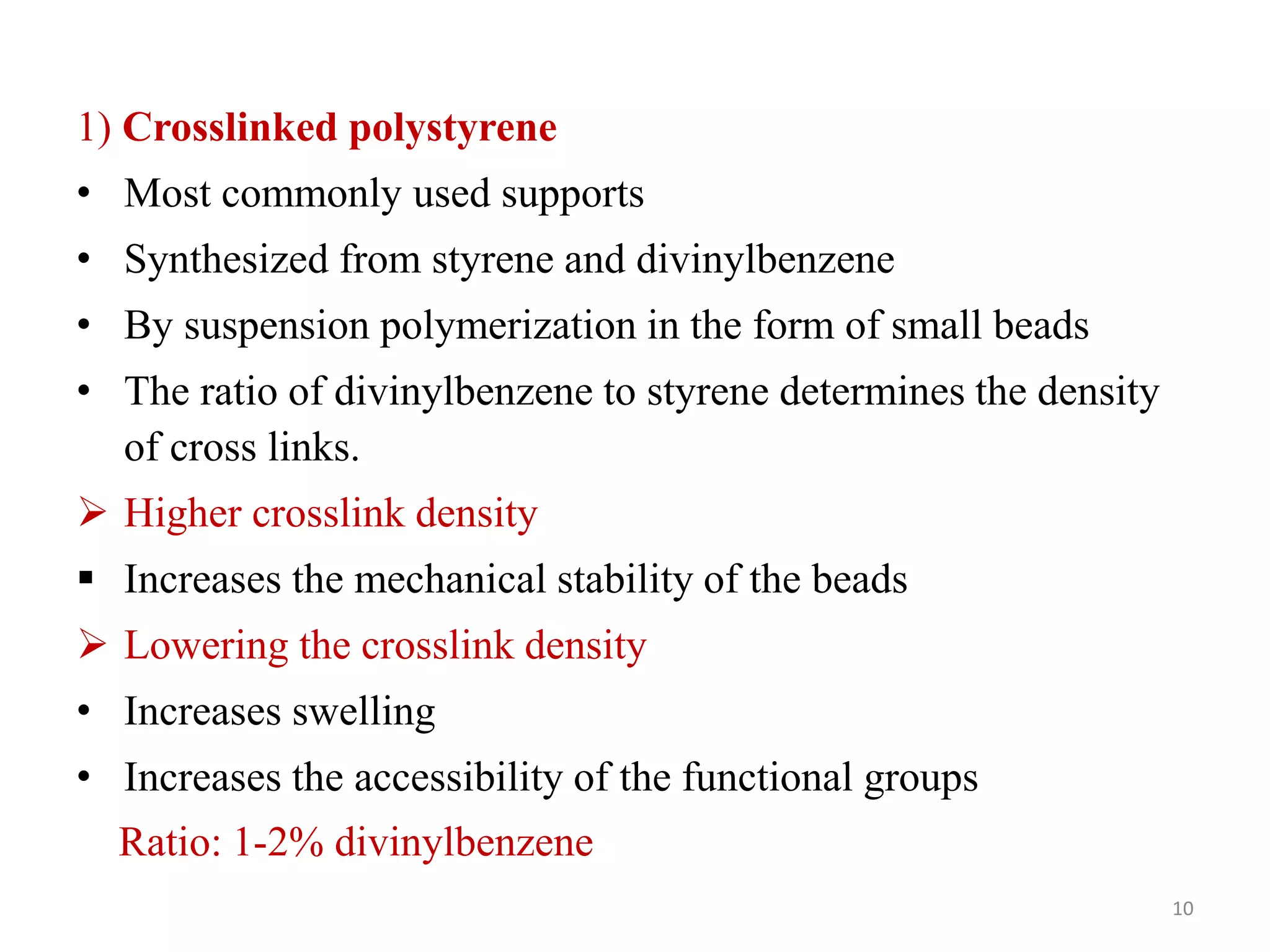
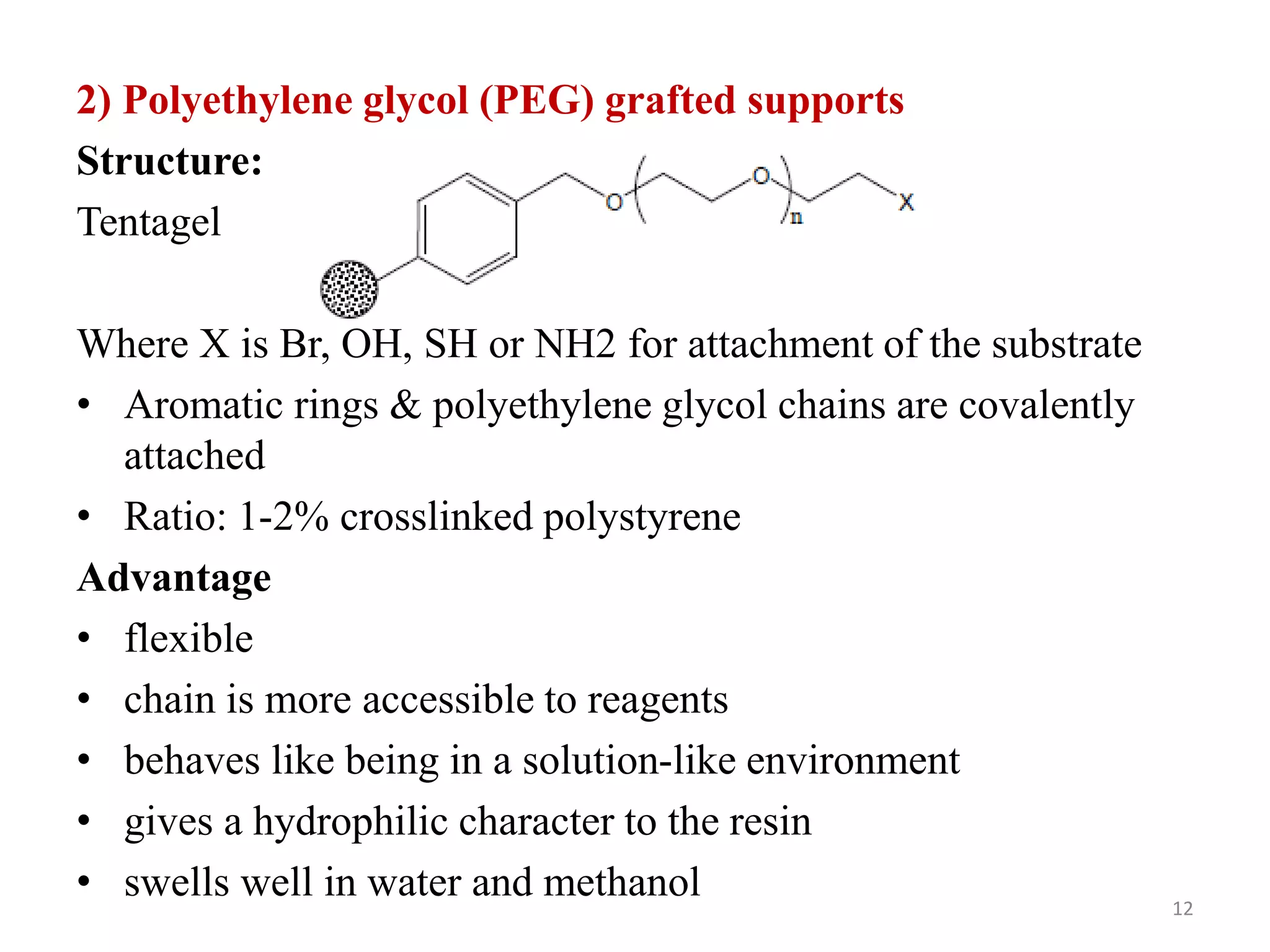
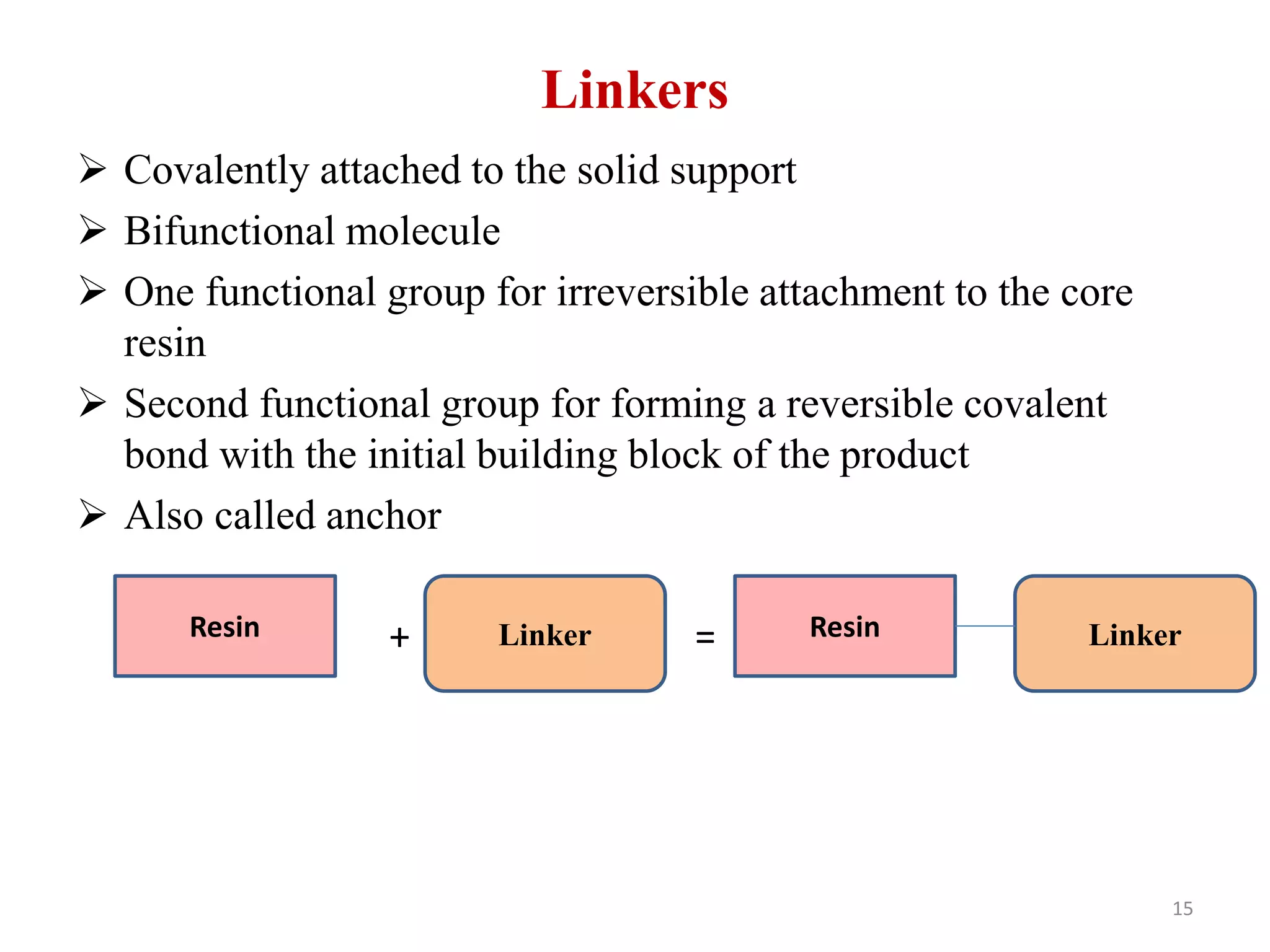
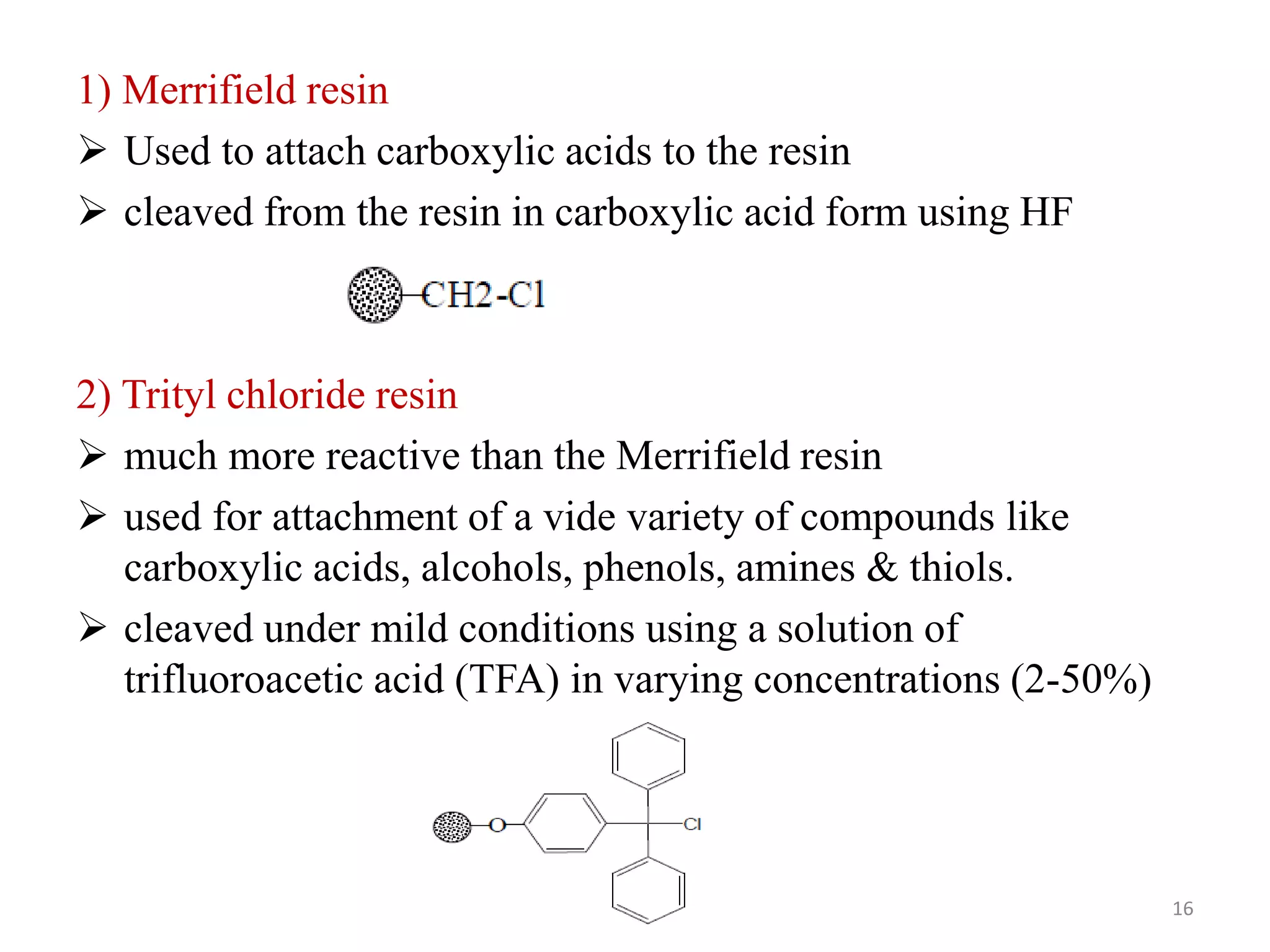
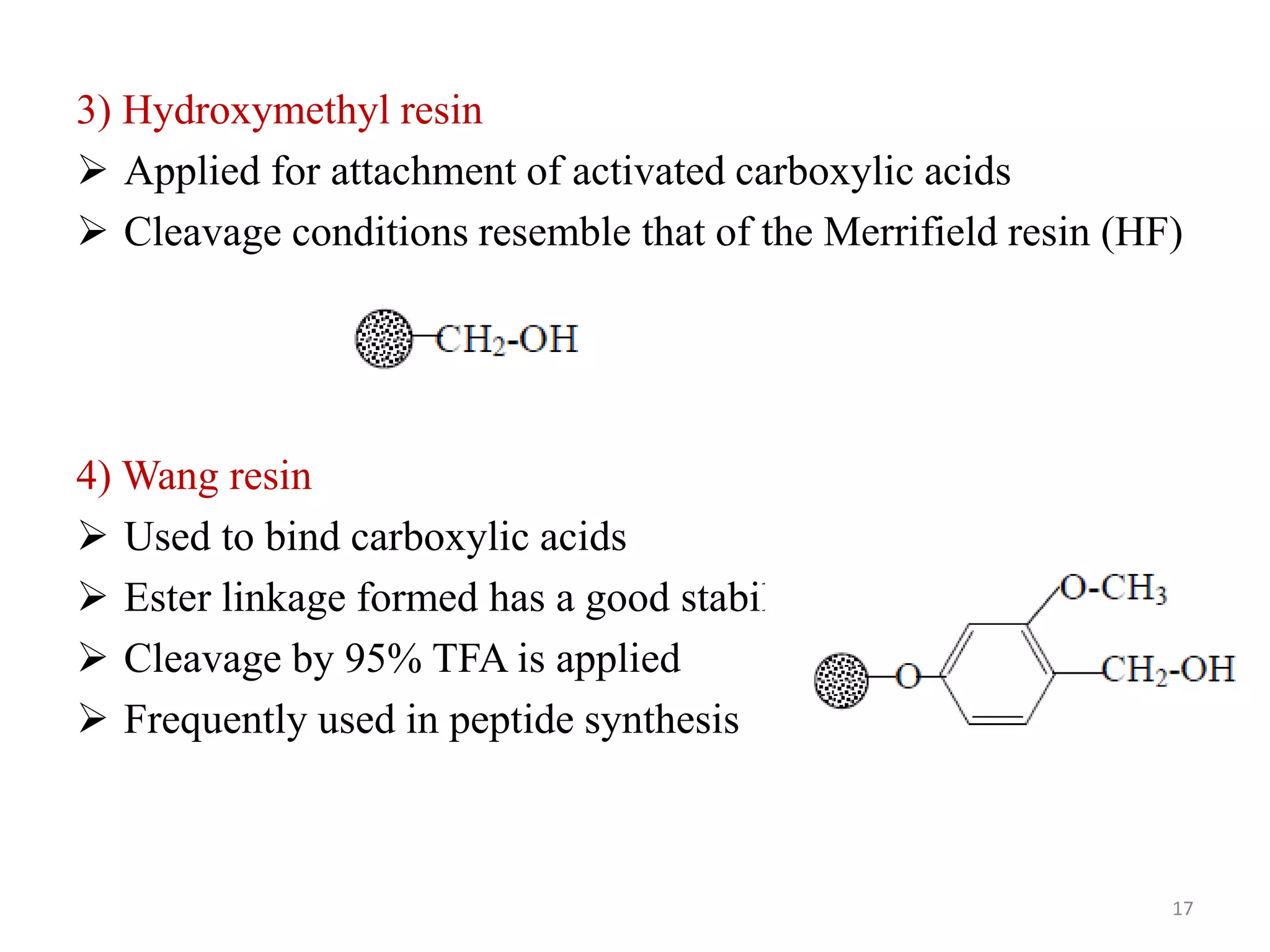
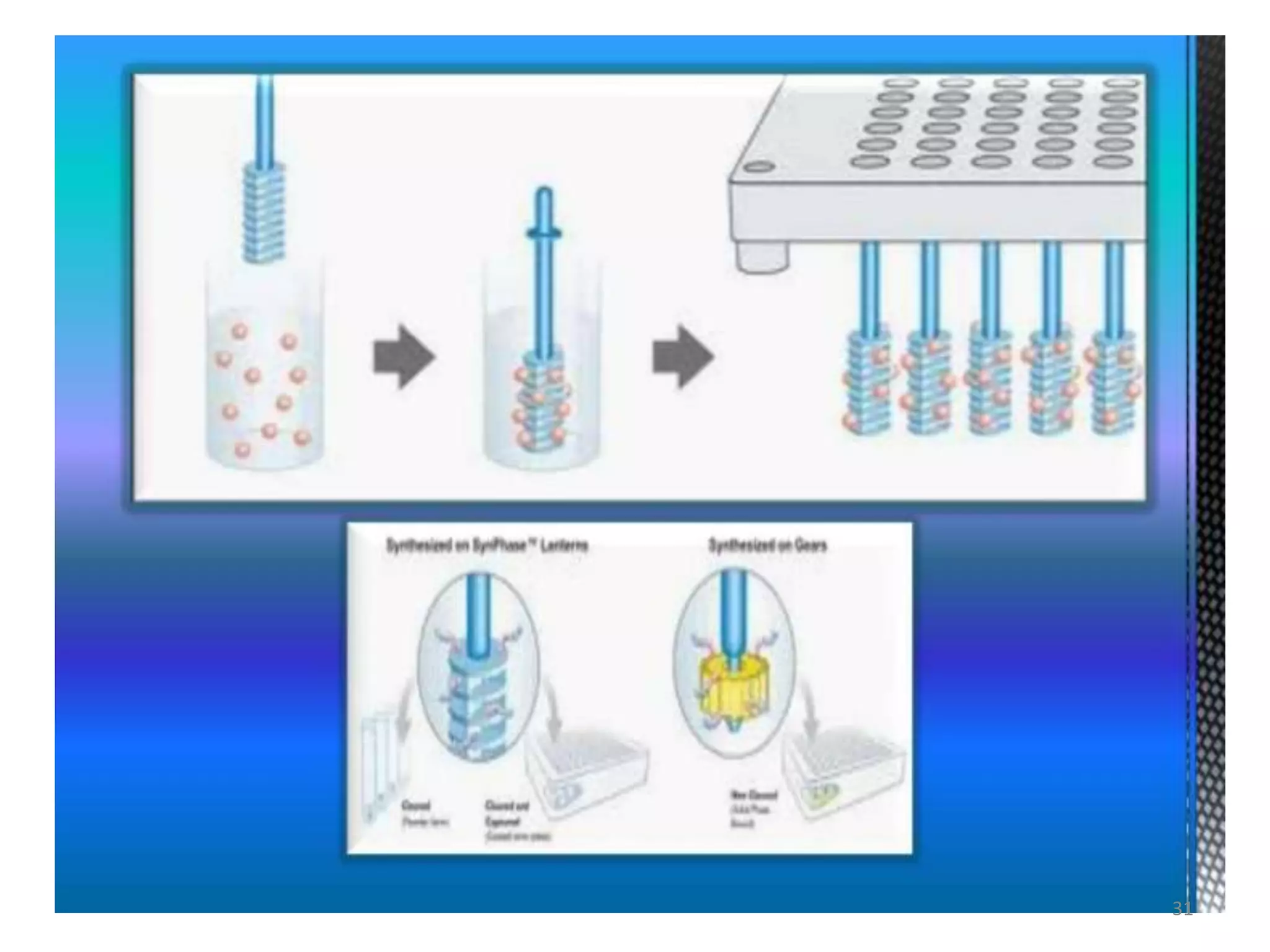
3) Combinatorial chemistry relies on solid supports like polystyrene or polyethylene glycol resin beads that are insoluble and compatible with reagents. Linkers attach initial building blocks like amino acids to these supports, and parallel