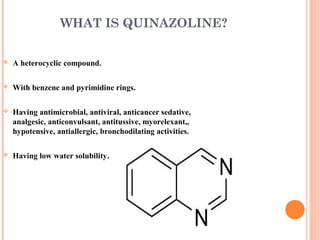
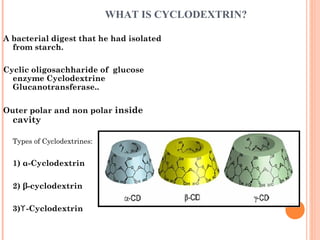
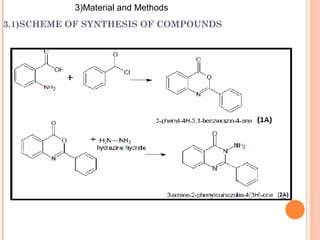
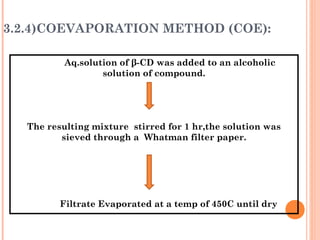
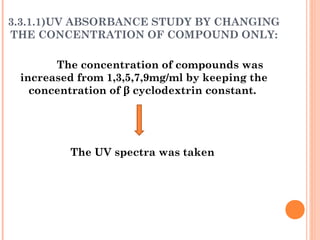
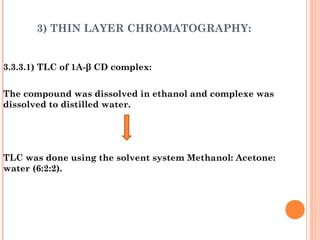
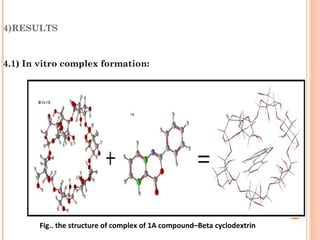
The document discusses the solubility enhancement and physicochemical characterization of inclusion complexes formed between quinazoline-4(3H)-ones and β-cyclodextrin. It outlines various methodologies for complex preparation, such as physical mixing and kneading, and characterizes the complexes using spectroscopic techniques. The study concludes that the kneading method is the most efficient for enhancing the solubility of the compounds in water.



![3.2)PREPARATIPON OF COMPLEX
3.2.1)In Silico complex formation:
By ChemDraw 8.0 software.
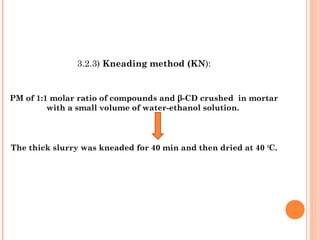
3.2.2) Physical mixture (PM) :
Compound and β-CD [1:1 molar ratio] taken together.
Crushed together for 40 min.](https://image.slidesharecdn.com/finalravisolubila-130728052157-phpapp01/85/Solubilisation-of-Quinazoline-drugs-by-using-Beta-cyclodextrin-complex-formation-7-320.jpg)



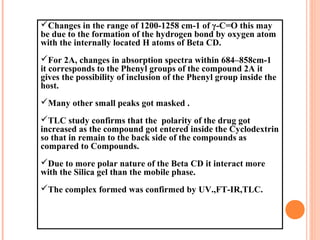
![3.3.2)FOURIER TRANSFORM INFRARED
SPECTROPHOTOMETRY [FT-IR]:
The FT-IR spectra of compounds`1A,2A, complexes of different compounds and
cyclodextrin were taken in Nujol and compared for the presence of different peaks in
the FT-IR spectra(Shimadzu FT-IR 8300).](https://image.slidesharecdn.com/finalravisolubila-130728052157-phpapp01/85/Solubilisation-of-Quinazoline-drugs-by-using-Beta-cyclodextrin-complex-formation-14-320.jpg)










![)
11) F. Schardinger, Bildung kristallisierter Polysaccharide (Dextrine) aus Stärkekleister durch Microben, Zentralbl.
Bakteriol. Parasitenkd. Abt. II 29 , 188–197-(1911)
12) [5] K. Freudenberg, R. Jacobi, Über Schardinger Dextrine aus Stärke Liebigs, Ann. Chem. 518 102–108. (1935)
(13) K.L. Larsen, Large cyclodextrins, J. Incl. Phenom. Macrocycl. Chem. 43 1–13-(2002)
(14) H. Ueda, T. Endo, Large-ring cyclodextrins, in: H. Dodziuk (Ed.), Cyclodextrins and their Complexes. Chemistry,
analytical methods, applications, Wiley-VCH Verlag, Weinheim, pp. 370–380-,(2006)
(15) F. Cramer, Einschlussverbindungen, Springer-Verlag, Berlin, (1954).
(16) K. Uekama (Ed.), Cyclodextrins in drug delivery, Adv. Drug Deliv. Rev., vol. 36,(1999).
(17) Subarna ganguli,et.al.One-pot Synthesis Of Novel Quinazoline Derivatives and Their Antimicrobial activity.
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 4, Issue 4, (2012).
18) Marcus E. Brewstera, Thorsteinn Loftsson, Cyclodextrins as pharmaceutical solubilizers Advanced Drug Delivery
Reviews 59 645–666.- (2007)
19) Rosa Iacovino, Jolanda Valentina Caso , Filomena Rapuano , Agostino Russo ,Marina Isidori , Margherita Lavorgna
Gaetano Malgieri. Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-
Cyclodextrin Inclusion Complex and Carla Isernia. Molecules 17, 6056-6070; doi:10.3390/molecules17056056-(2012),
20) S. Tavornvipas, F. Hirayama, H. Arima, K. Uekama, T. Ishiguro, M. Oka, K. Hamayasu, H. Hashimoto, 6-O-α-(4-O-α-D-
glucoronyl-D-glucosyl-β- cyclodextrin: solubilizing ability and some cellular effects, Int. J. Pharm. 249 199–209 (2002)
21) A.T.H.J.DeBie,B. vanOmmen,A.Bär,Disposition of 14C-γ-cyclodextrin in germ-free and conventional rats, Regul.
Toxicol. Pharmacol. 27 ,150–158 (1998)
22) B. Van Ommen, A.T.H.J. De Bie, A. Bär, Disposition of 14C-α- cyclodextrin in germ-free and conventional rats, Regul.
Toxicol. Pharmacol. 39, S57–S66-(2004)
23) G. Antlsperger, New aspects in cyclodextrin toxicology, in: A.R. Hedges(Ed.), Minutes of the sixth international
symposium on cyclodextrins, Editions de Santé: Paris, pp. 277–283-(1992),
24) K. Matsuda, Y. Mera, Y. Segawa, I. Uchida, A. Yokomine, K. Takagi, Acute toxicity study of γ cyclodextrin (γ-CD) in
mice and rats, Ogo Yakuri (Pharmacometrics), 26,287–291-(1983)
25) A.C. Moffat, M.D. Osselton, B. Widdop (Eds.), 3rd ed., Clarke's Analysis of drugs and poisons, vol. 2, Pharmaceutical
Press, London, (2004).
26) Nagarsenker MS, and Joshi MS. Celecoxib-cyclodextrin systems: characterization and evaluation of in vitro and in
vivo advantage. Drug Dev Ind Pharm.;31:169-178-(2005)
27) Tozuka Y, Wongmekiat A, Sakata K, Moribe K, Oguchi T, and Yamamoto K. Cogrinding with cyclodextrin as a
nanoparticle preparation method of a poorly water soluble drug. J Incl Phenom.;50:67-71-(2004)](https://image.slidesharecdn.com/finalravisolubila-130728052157-phpapp01/85/Solubilisation-of-Quinazoline-drugs-by-using-Beta-cyclodextrin-complex-formation-28-320.jpg)


